Minor Bone Augmentation
Scientific background
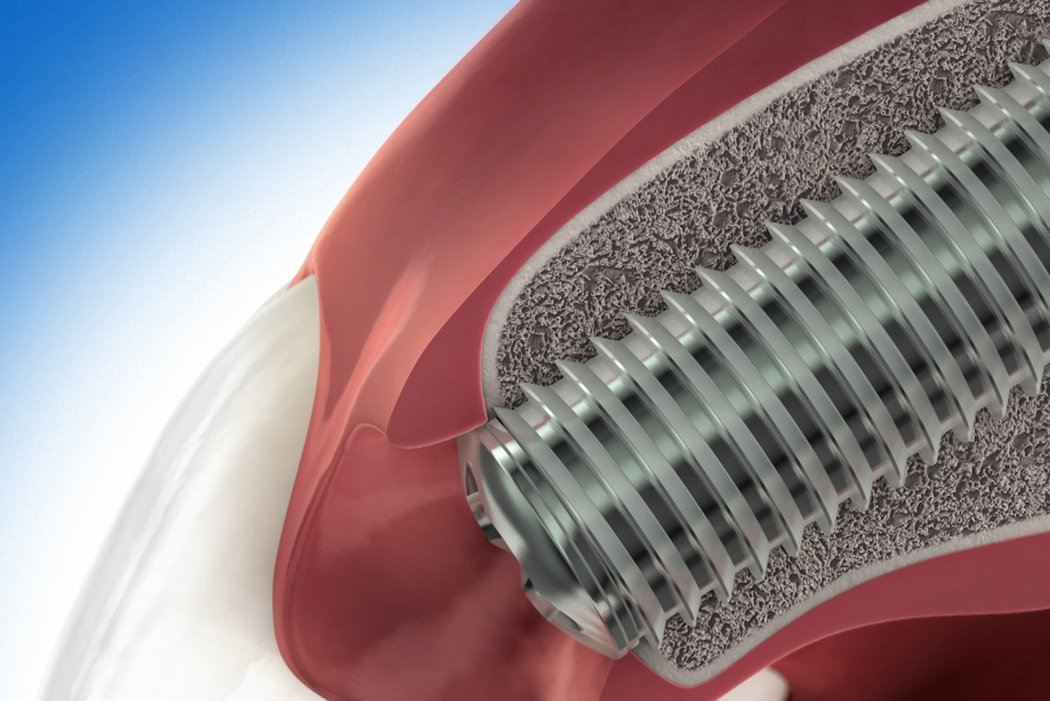
Guided bone regeneration (GBR) is a surgical procedure that uses grafting materials and barrier membranes to stimulate and direct the growth of new bone at defect sites.
Autologous bone, and/or a biomaterial, is placed into a bone deficient area, maintaining space and stimulating formation of new bone. Barrier membranes covering the filled defect prevent the ingrowth of soft tissue.
GBR approaches are used, for example, to restore bone in case of fenestration or dehiscence defects around an implant, to compensate for major jawbone deficiencies or to avoid bone resorption after tooth extraction in deficient alveoli.
Why use a bone substitute instead of autologous bone?
Autologous bone best stimulates new bone formation and therefore seems to be the preferred grafting material. Harvesting patient bone, however, causes pain and prolongs surgery and recovery time. In addition, autogenous bone is subject to a certain degree of resorption1.
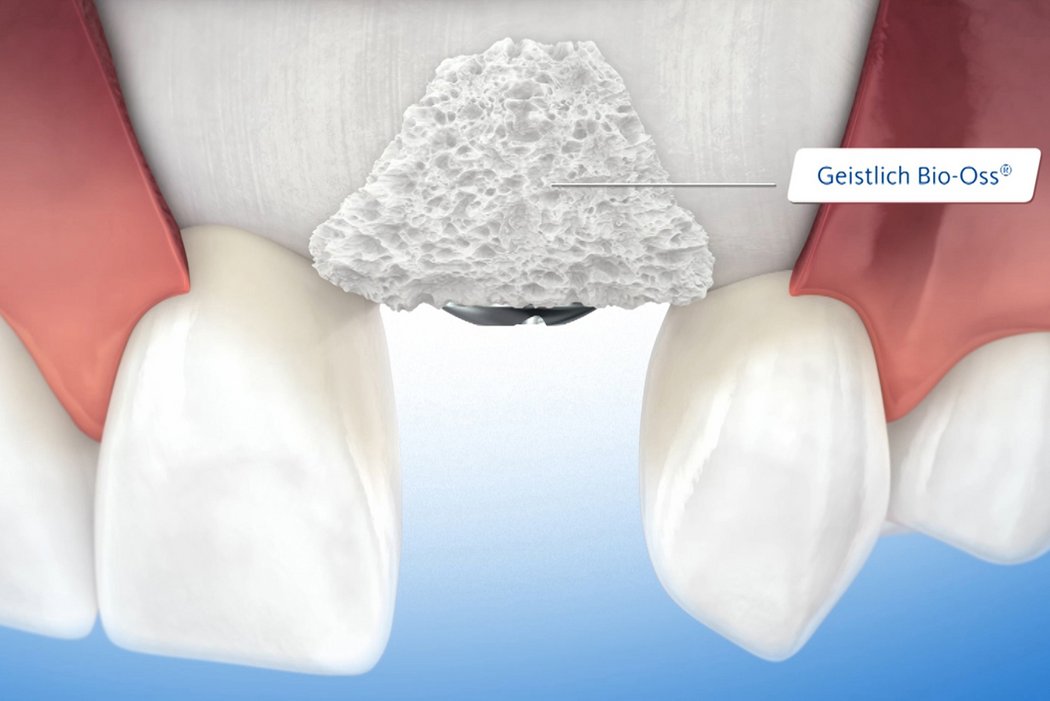
Biomaterials are a valuable alternative to autologous bone and show several advantages. Geistlich bone substitute materials spare the patient painful bone harvesting, offer a stable scaffold for bone formation and help to attain long-term volume stability due to their low resorption rate2. For these reasons, Geistlich Bio-Oss® is the most frequently used bone substitute material in dentistry3,4.
Why use a barrier membrane?
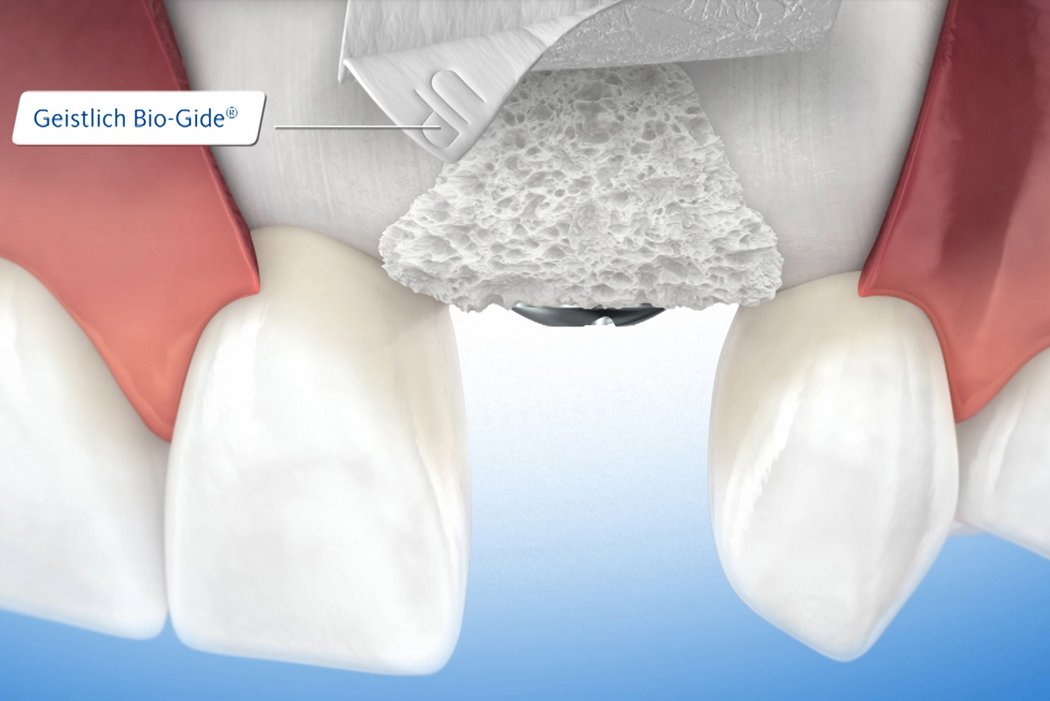
Use of a collagen membrane ensures optimal wound healing and bone regeneration7,8,20-28. More than 20 years of clinical experience have shown that a native collagen membrane such as Geistlich Bio-Gide® provides a temporary barrier21,27,29, and ensures optimal tissue integration7,21 and heals largely without complications7,9,20. Surgical removal is unnecessary as the membrane is resorbed by the body.
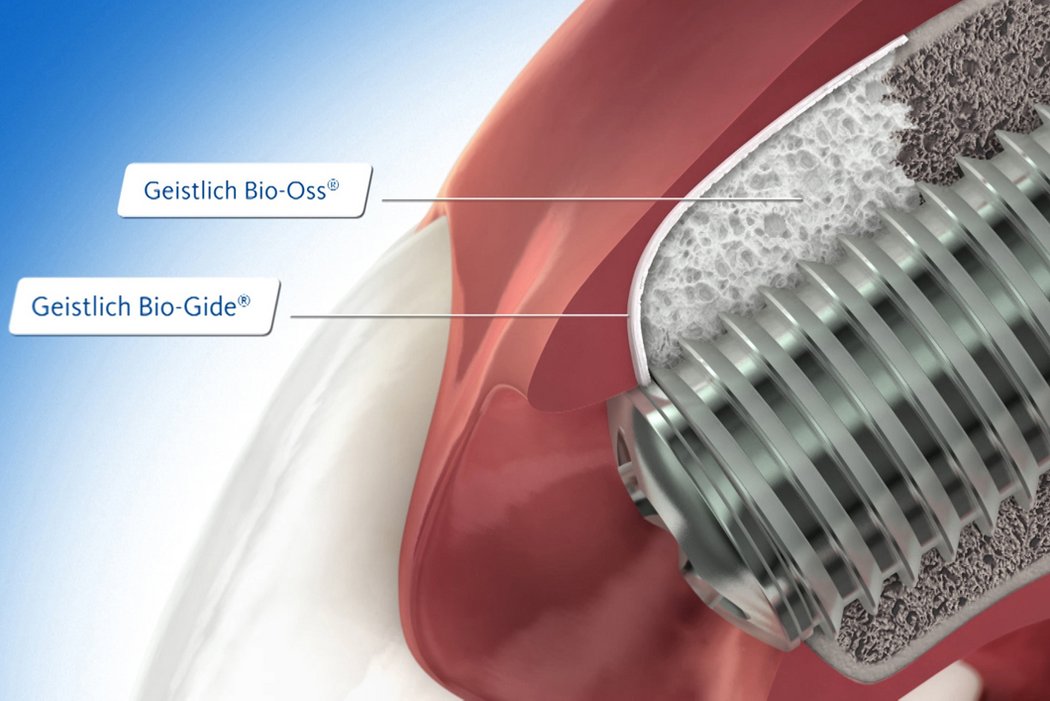
Geistlich Bio-Oss® and Geistlich Bio-Gide® are the most frequently used products in regenerative procedures such as minor bone augmentation3,4.
They ensure
- Reliable bone formation7,10-15,21
- High level of osseointegration and long-term volume preservation12,16,17
- Long-term implant survival rate18
- Complication-free healing7,9,20
- Excellent aesthetic outcome19,30
Geistlich biomaterials are reliable and facilitate procedures even for less experienced surgeons.
References:
- Jensen T et al., Clin Oral Implants Res. 2012 Mar;23(3):263–73 (Clinical study)
- Orsini G et al.,Oral Dis. 2007, Nov;13(6):586–93 (Clinical study)
- Millennium Research Group, Dental Biomaterials North America, 2018 (Market research)
- Millennium Research Group, Dental Biomaterials Europe, 2018 (Market research)
- Perelman-Karmon M et al., Int J Periodontics Restorative Dent.2012 Aug;32(4):459–65 (Clinical study)
- Wallace SS et al., Int J Periodontics Restorative Dent. 2005 Dec;25(6):551-9 (Clinical study)
- Schwarz F, et al.: Clin Oral Implants Res 2006; 17: 403-09 (Preclinical Study)
- Schwarz F, et al.: Clin Oral Implants Res 2008; 19(4): 402-15 (Preclinical Study)
- Becker J, et al.: Clin. Oral Implants Res 2009; 20(7): 742-93 (Clinical study)
- Aghaloo TL et al., Int J Oral Maxillofac Implants. 2007; 22(suppl):49–70 (Clinical study)
- Orsini G et al., J Biomed Mater Res B Appl Biomater. 2005 Jul(1);448–57 (Clinical study)
- Piattelli M et al., Int J Oral Maxillofac Implants.1999 Nov–Dec;14(6):835–40 (Clinical study)
- Orsini G et al.,Oral Dis. 2007, Nov;13(6):586–93 (Clinical study)
- Traini T et al., J Periodontol. 2007 May; 78(5):955–61 (Clinical study)
- Degidi M et al., Clin Implant Dent Relat Res. 2009 Sep;11(3):178-82 (Clinical study)
- Sartori S, et al., Clin Implants Res. 2003 Jun;14(3):369–72 (Clinical study)
- Maiorana C, et al. Int J Periodontics Restorative Dent. 2005 Feb;25(1):19–25 (Clinical study)
- Jung R et al., Clin Oral Implants Res. 2013 Oct;24(10):1065–73 (Clinical study)
- Buser D et al., J Periodontol. 2011 Mar;82(3):342–9 (Clinical study)
- Tal H et al. Clin Oral Implants Res. 2008; 19(3) : 295-302. (Clinical study)
- Rothamel D et al. Clin. Oral Implants Res. 2005; 16(3): 369-378. (Pre-clinical study)
- Kim M et al. In Vivo. 2008; 22(2):231-6. (Pre-clinical study)
- Zitzmann NU et al. Int J Oral Maxillofac Implants.12, 1997;844-852. (Clinical study)
- Rothamel D et al. Clin. Oral Implants Res. 2004;15:443-449. (Pre-clinical study)
- Hämmerle CH and Karring T. Periodontol. 2000. 1998;17:151-175. (Expert opinion)
- Hämmerle CH et al. Clin. Oral Implants Res. 2008;19:18-25. (Clinical study)
- Gielkens PFM et al. Clin. Oral Implants Res. 2008;19:516-521. (Pre-clinical study)
- Pjetursson BE et al. J. Clin. Periodontol. 2008;35:216-240. (Clinical study)
- Rothamel D et al. Int J Oral Maxillofac Implants. 2012 Jan–Feb;27(1):146–54. (Pre-clinical study)
- Buser D et al. J Dent Res. 2013 Dec;92(12 Suppl):176S–82S. (Clinical study)
Clinical Cases
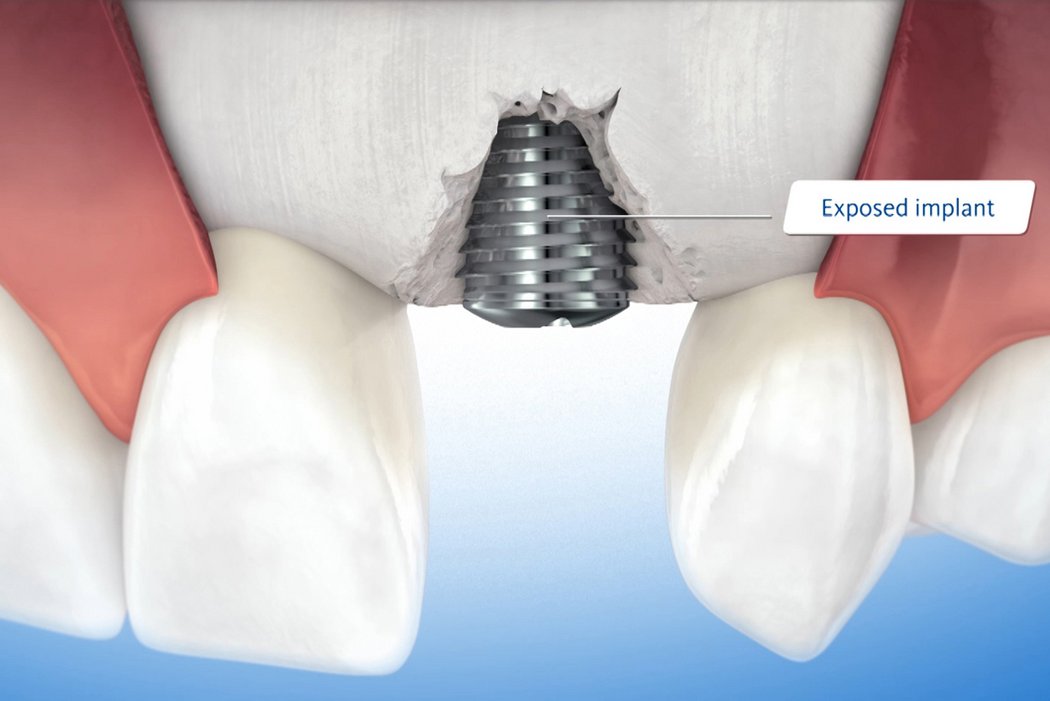
Dehiscence defects around implants
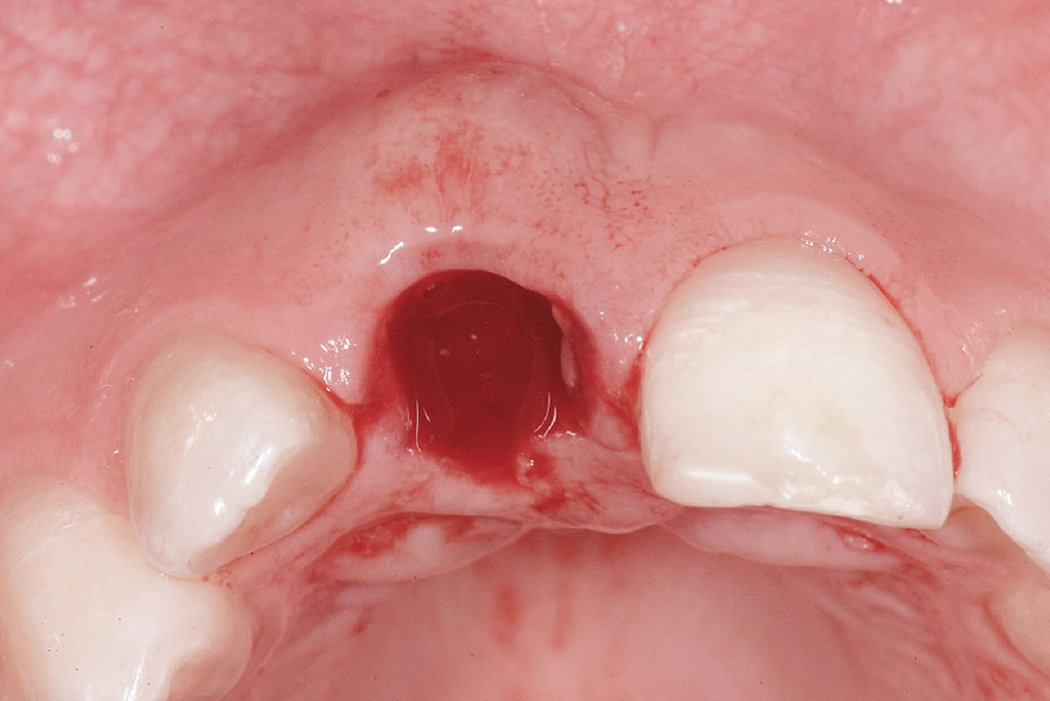
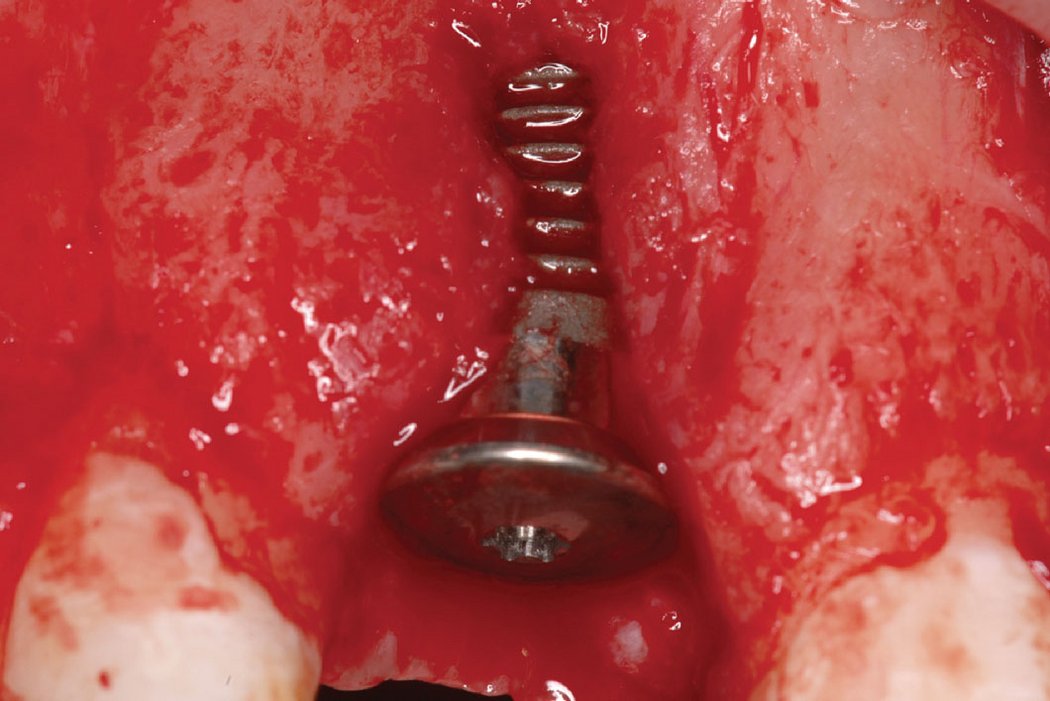
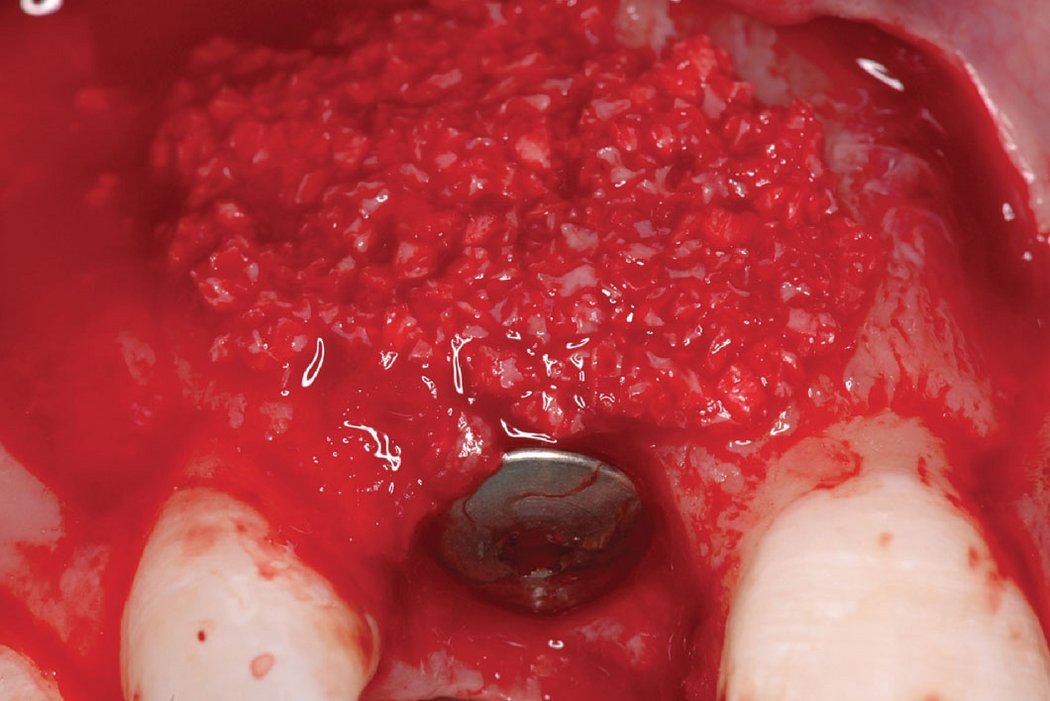
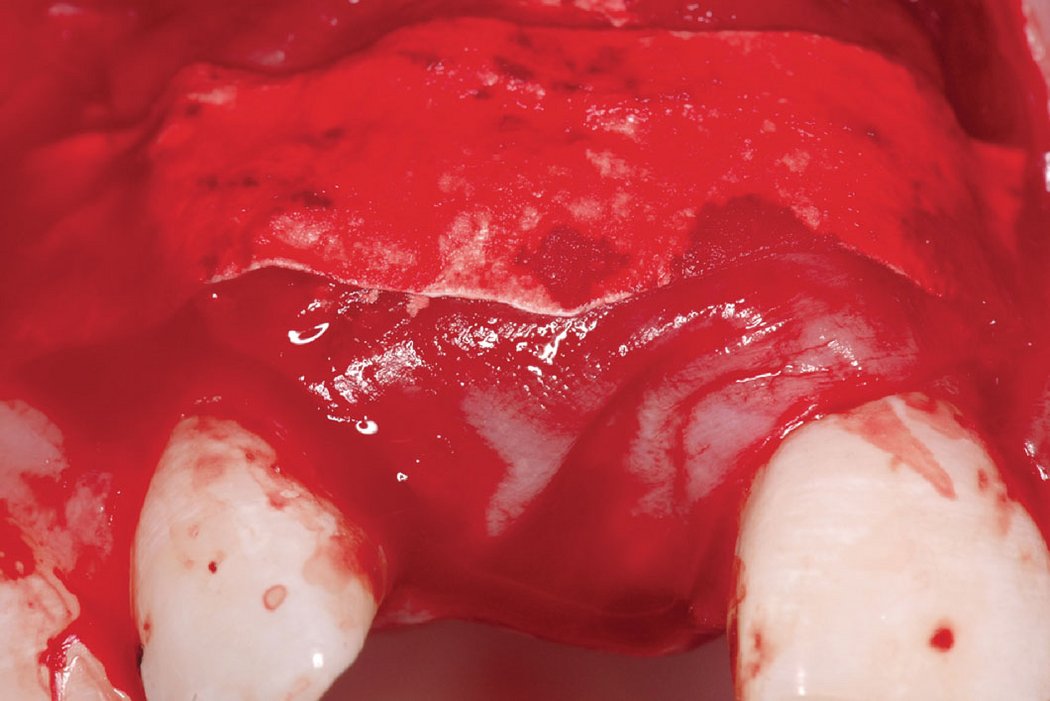
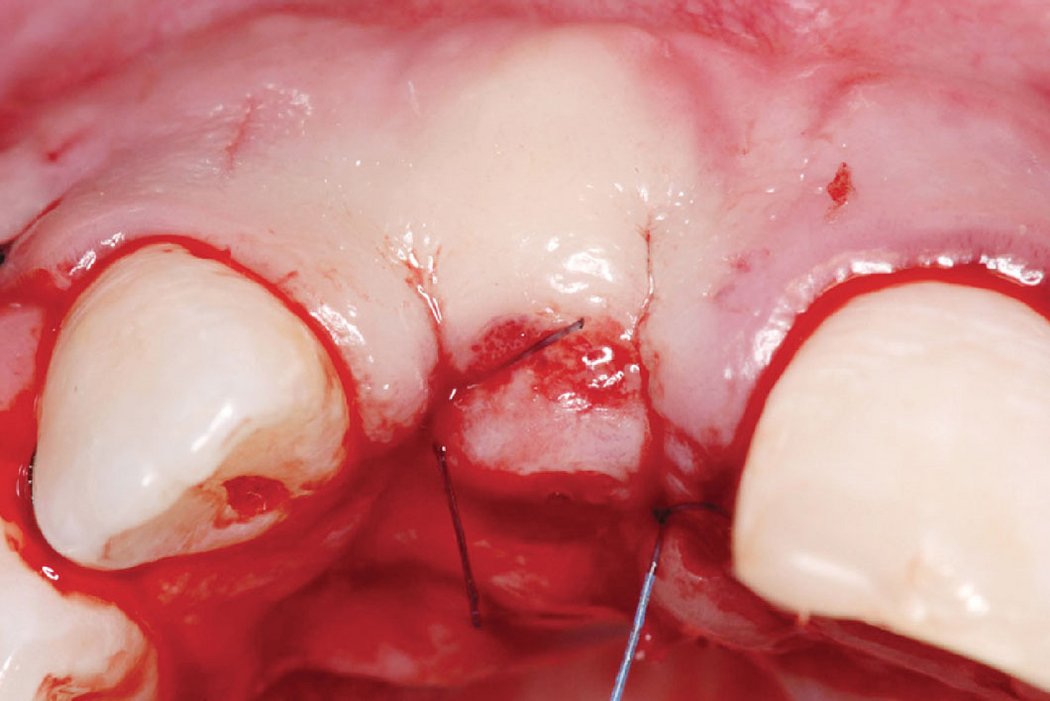
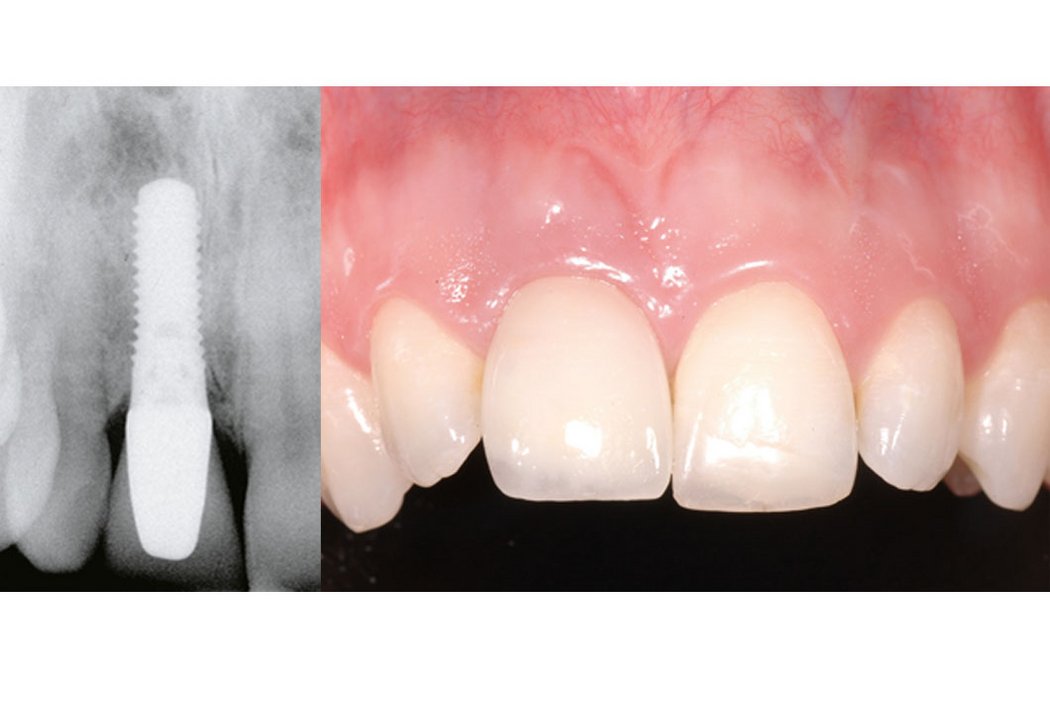
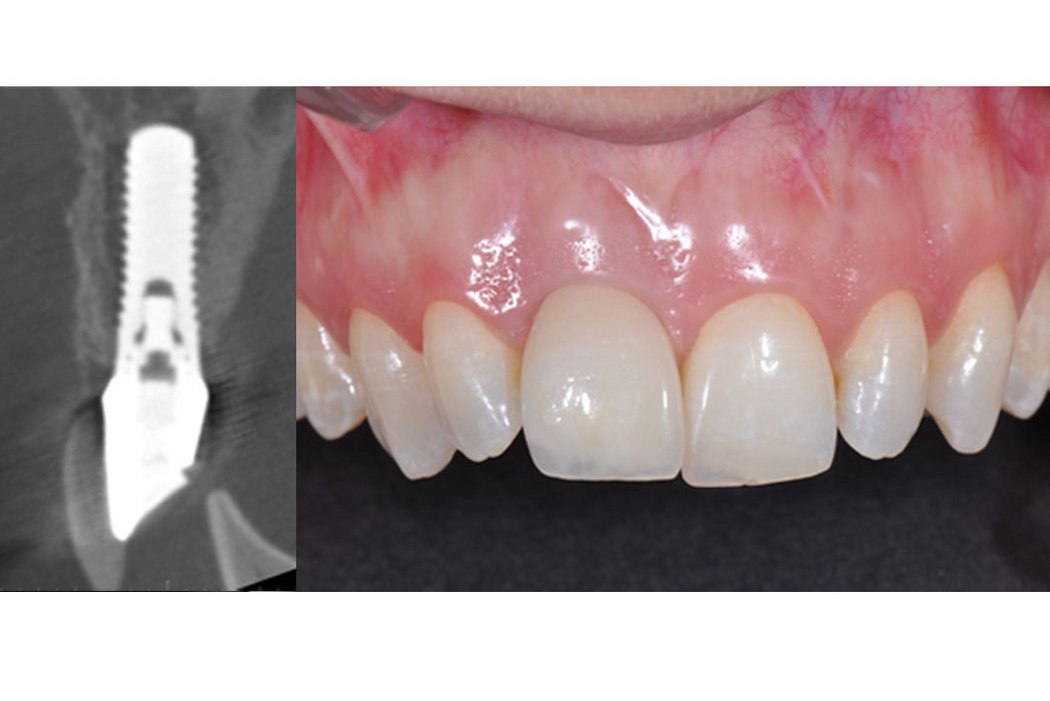
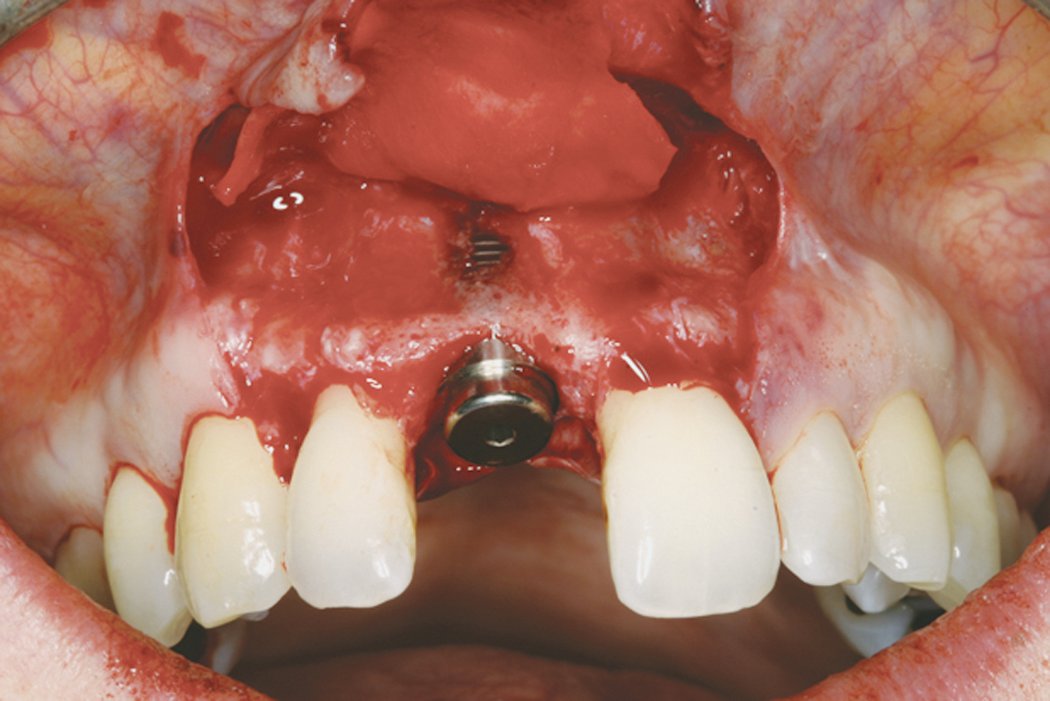
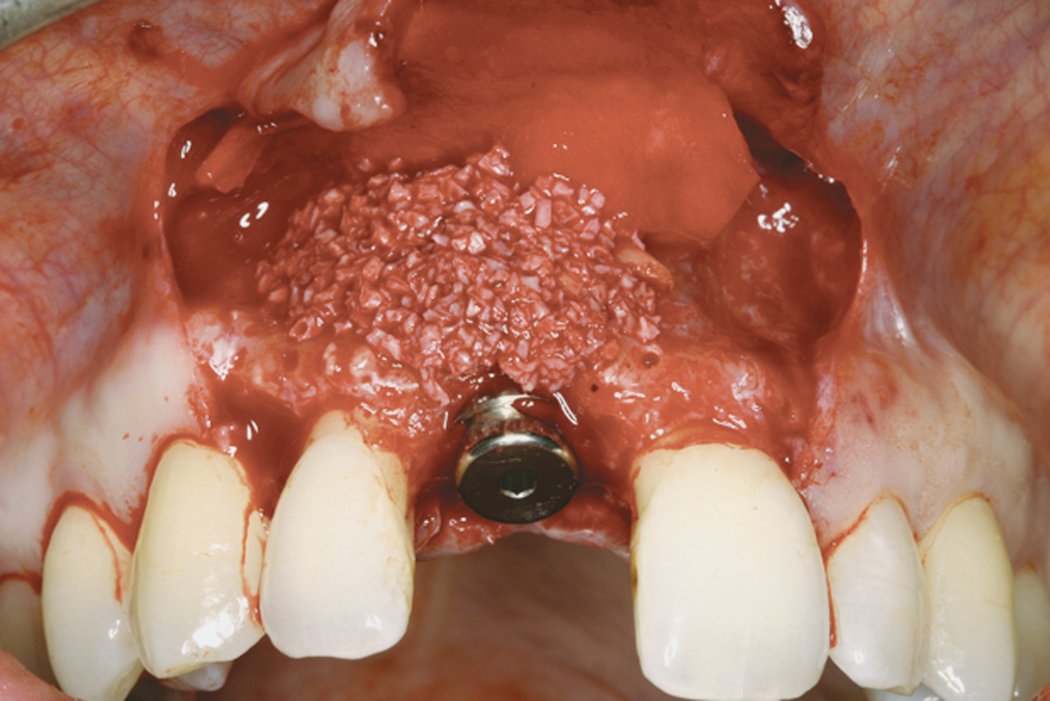
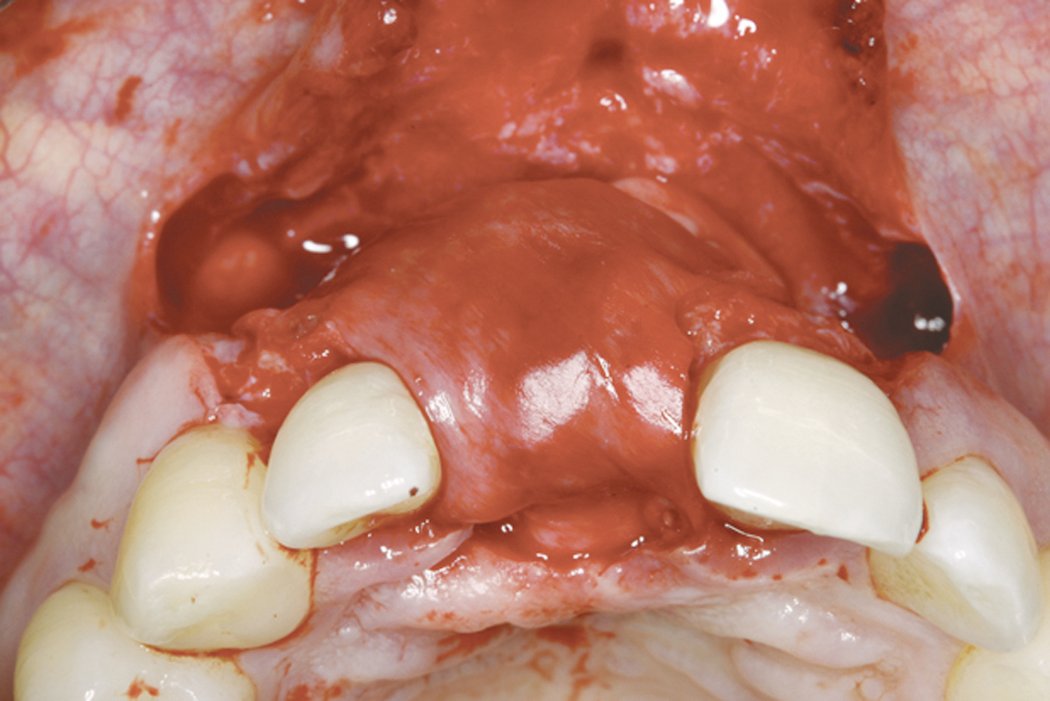
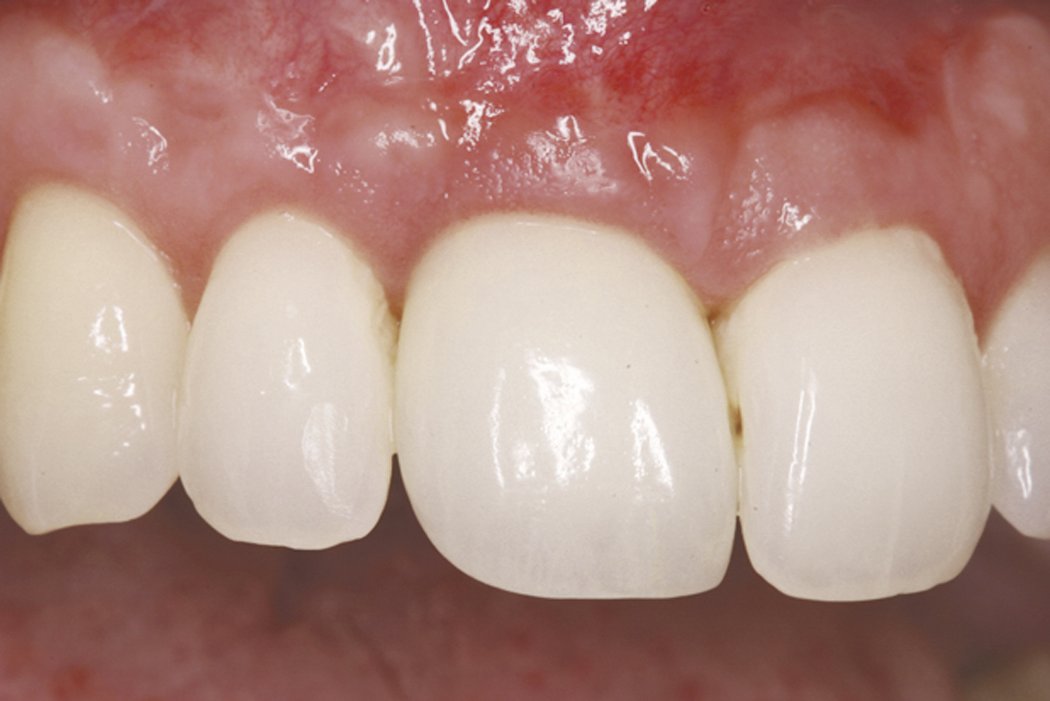
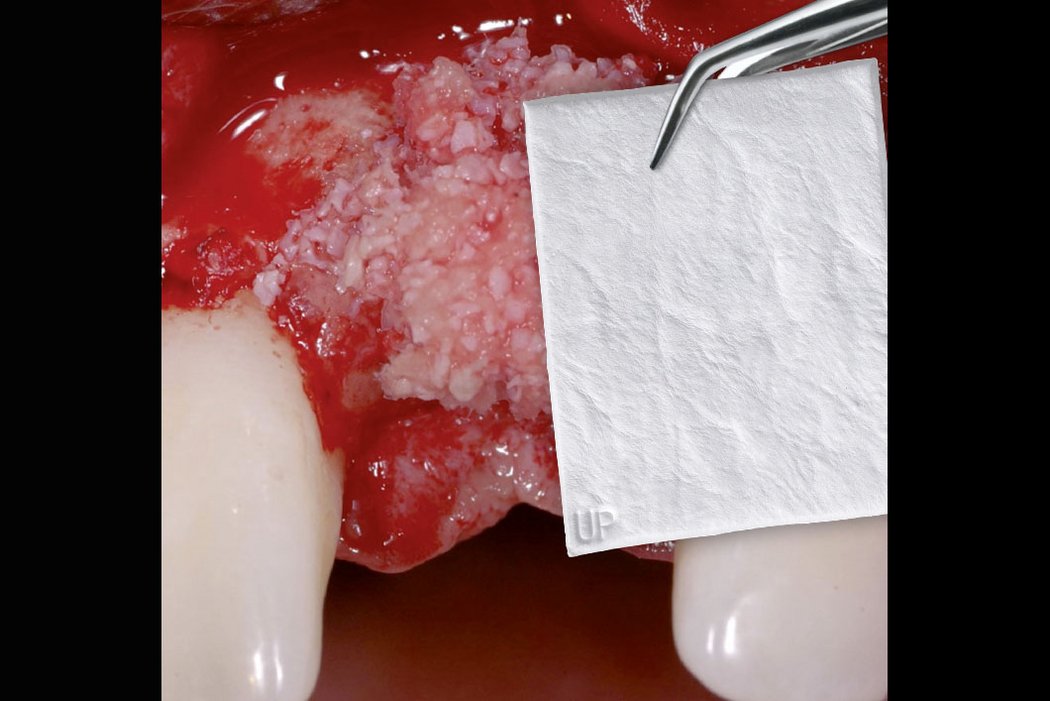
Clinical case: Minor bone augmentation performed simultaneously with implant placement four to six weeks after tooth extraction favours the long-term aesthetic appearance.
Geistlich biomaterials help reconstruct buccal dehiscence at the time of implant placement for a stable long-term result.
References:
- Rothamel D et al. Clin. Oral Implants Res. 2005; 16(3): 369-378. (Pre-clinical study)
- Gielkens PFM et al. Clin. Oral Implants Res. 2008;19:516-521. (Pre-clinical study)
- Rothamel D et al. Int J Oral Maxillofac Implants. 2012 Jan–Feb;27(1):146–54. (Pre-clinical study)
Fenestration defects around implants
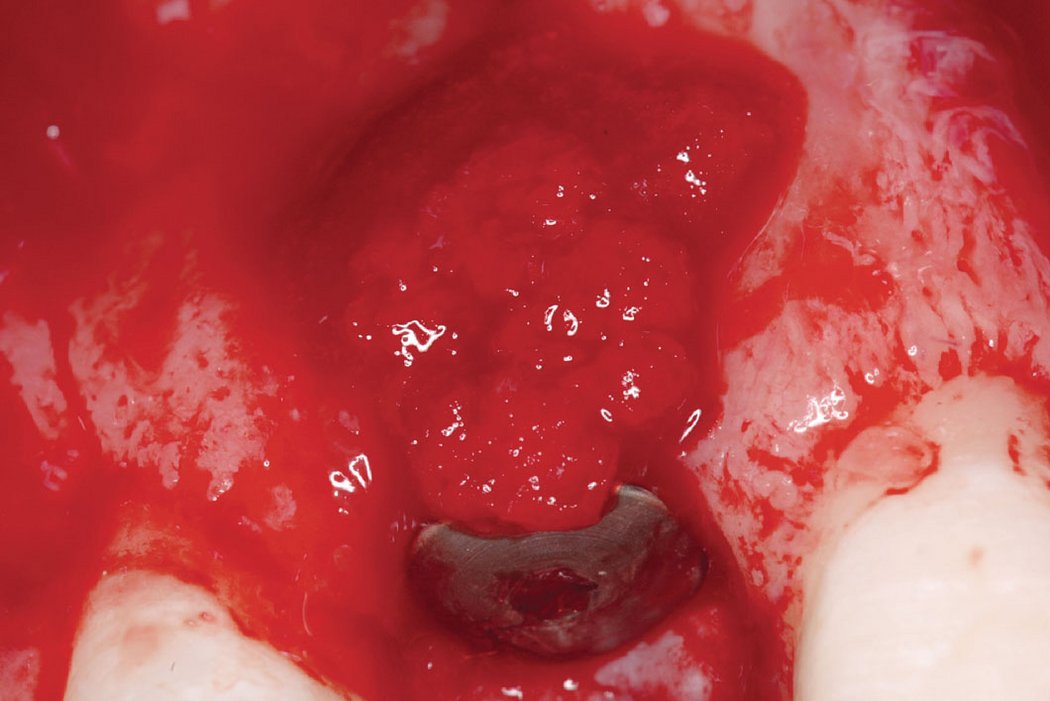
Clinical case: A fenestration defect is augmented at an anterior implant site.
Successful hard and soft tissue healing of a peri-implant defect using the GBR technique with Geistlich Bio-Oss® and Geistlich Bio-Gide®.