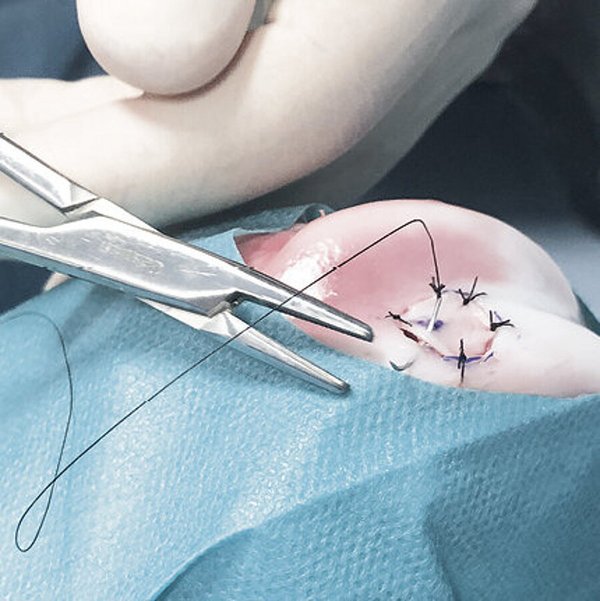
AMIC® Chondro-Gide® in der Hüfte
Chondrale Defekte in der Hüfte aufgrund von Traumata, Osteonekrose, Labralrissen und weiteren Ursachen können zu gravierenden Dysfunktionen und Gelenkschmerzen führen. Das Femoroazetabuläre Impingement (FAI) ist eine weitere häufige Ursache für lokalisierte Knorpeldefekte und Schäden, die eine Hüftarthroskopie bedingen1.
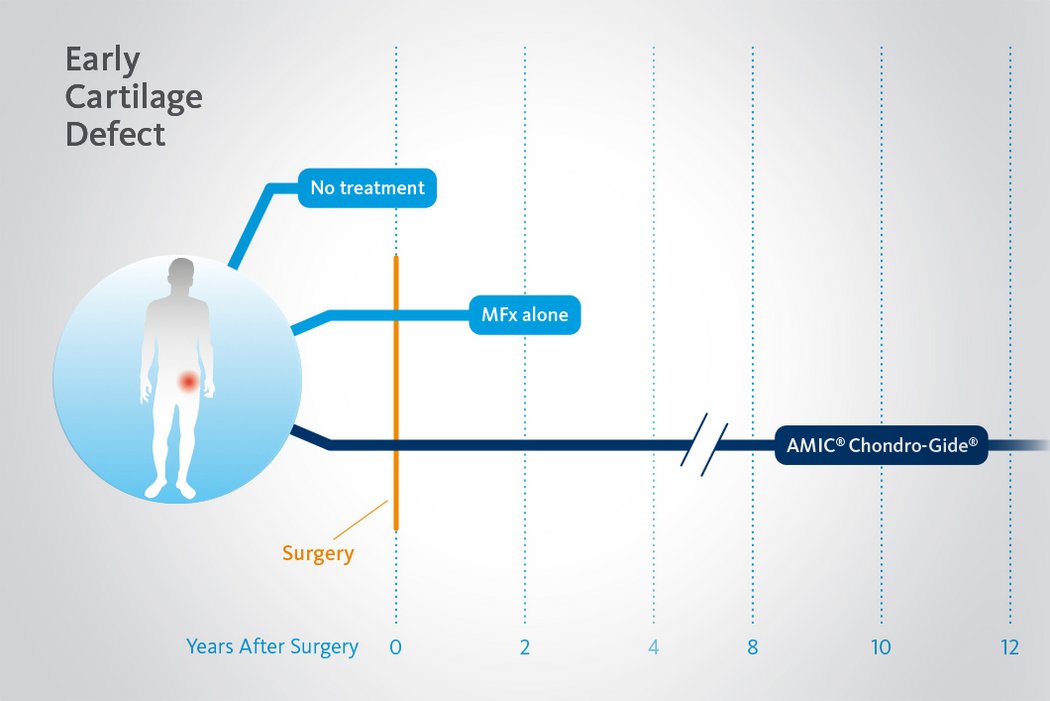
Das Verzögern oder Verhindern eines kompletten Hüftersatzes ist heute möglich
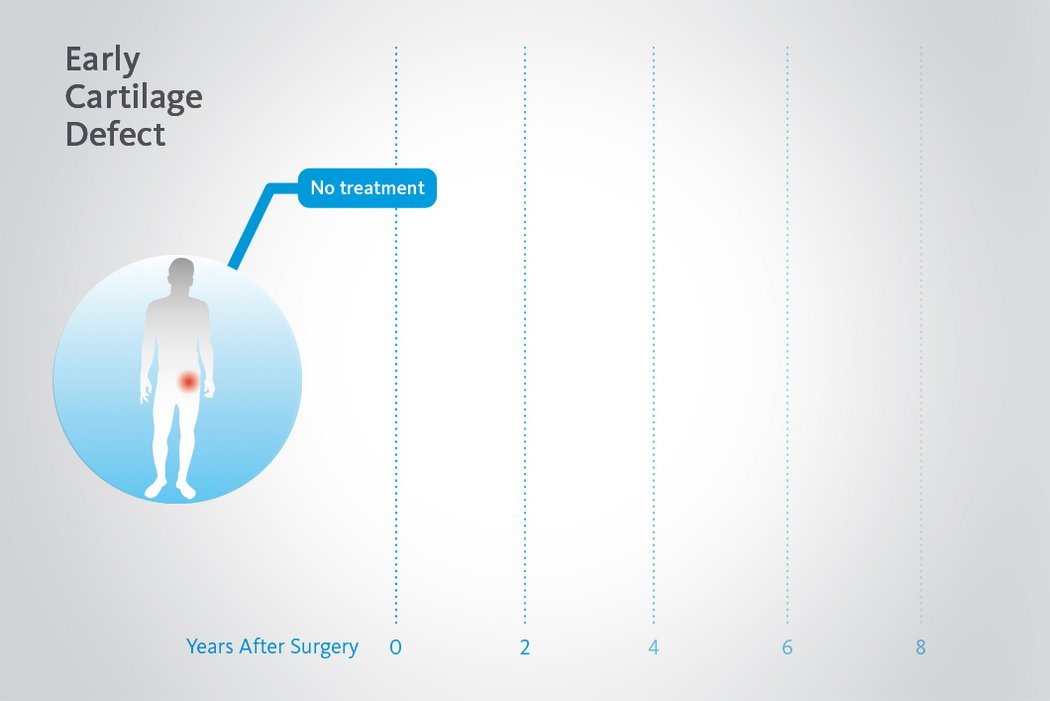
Beschädigtes Knorpelgewebe hat eine begrenzte Selbstheilungskraft. Wenn Knorpeldefekte in der Hüfte nicht adäquat behandelt werden, kann der Prozess fortschreiten. Dank minimalinvasiven arthroskopischen Behandlungsmethoden für chondrale Defekte in der Hüfte ist es nun möglich Hüftgelenksknorpel zu erhalten und einen kompletten Gelenkersatz1 zu verzögern oder allenfalls sogar ganz zu vermeiden.
AMIC® Chondro-Gide® für die wirksame Behandlung von Knorpelläsionen
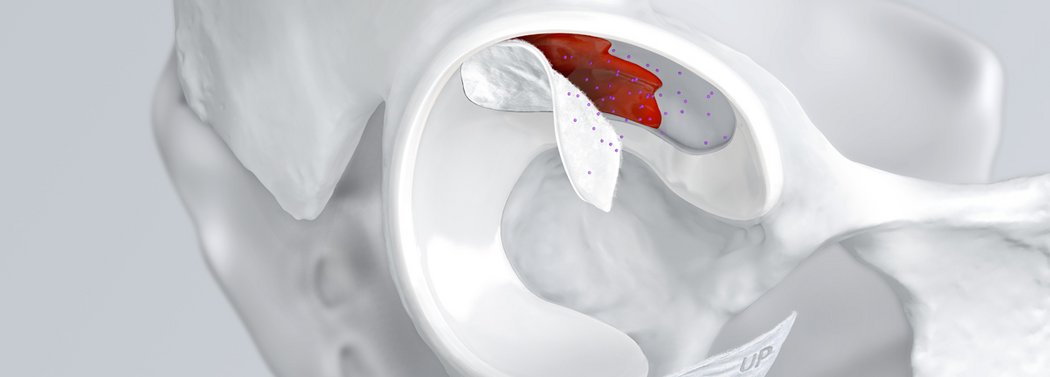
AMIC® Chondro-Gide® ist ein minimalinvasives einzeitiges Verfahren, das Knochenmarkstimulation in Kombination mit Chondro-Gide® nutzt, um Knorpeldefekte jeder Grösse zu reparieren.
AMIC® Chondro-Gide® wurde von Geistlich Surgery in Zusammenarbeit mit führenden europäischen Chirurgen entwickelt und ist eine effektive und kostengünstige Behandlung2,3 zur Reparatur von Knorpelläsionen, zur Schmerzprophylaxe und -linderung sowie zur Verlangsamung des Knorpelabbaus.
Mit seiner speziell entwickelten Bilayer-Struktur bietet Chondro-Gide® ein schützendes Umfeld, welches das Wachstum von neuem Knorpel unterstützt.4,5
- Biotechnisch gewonnene, Bilayer Kollagen I/III Membran4
- Biokompatibel und natürlich resorbierbar4
- Einfache Handhabung – biegsam und reissfest4
- Kann geklebt oder genäht werden4
- Kompatibel mit verschiedenen Gewebe-Regenerationstechniken6
- Einzeitiges Verfahren4
- Jederzeit verfügbar und einsatzbereit4
References
- MARQUEZ-LARA, A. et al., 2016, Arthroscopic Management of Hip Chondral Defects: A Systematic Review of the Literature. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2016. Vol. 32, no. 7, p. 1435-1443. DOI 10.1016/j.arthro.2016.01.058. Elsevier BV (Review).
- SCHIAVONE PANNI, A., et al. Good clinical results with autologous matrix-induced chondrogenesis (AMIC) technique in large knee chondral defects. Knee Surg Sports Traumatol Arthrosc, 2018 Apr 26(4):1130-36 (Clinical study).
- WALTHER, M., et al. Scaffold based reconstruction of focal full thickness talar cartilage defects. Clinical Research on Foot & Ankle, 2013, 1-5. (Clinical study).
- Geistlich Pharma AG data on file (Pre-clinical Study)
- GILLE, J., et al. Cell-Laden and Cell-Free Matrix-Induced-Chondrogenesis versus Microfracture for the Treatment of Articular Cartilage Defects: A Histological and Biomechanical Study in Sheep. Cartilage OnlineFirst, January 7, 2010, doi:10.1177/1947603509358721 (Pre-clinical study)
- KRAMER, J., et al. In vivo matrix-guided human mesenchymal stem cells. Cell Mol Life Sci, Mar 2006, 3(5), 616-626. (Clinical study)
- FONTANA, A. and DE GIROLAMO, L., 2015, Sustained 5-year benefit of autologous matrix-induced chondrogenesis for femoral acetabular impingement-induced chondral lesions compared with microfracture treatment. The Bone & Joint Journal. 2015. Vol. 97-B, no. 5, p. 628-635. DOI 10.1302/0301-620x.97b5.35076. British Editorial Society of Bone & Joint Surgery (Clinical study).
- DE GIROLAMO, L., et al., Autologous Matrix-Induced Chondrogenesis (AMIC) and AMIC Enhanced by Autologous Concentrated Bone Marrow Aspirate (BMAC) Allow for Stable Clinical and Functional Improvements at up to 9 Years Follow-Up: Results from a Randomized Controlled Study. Journal of Clinical Medicine. 2019. Vol. 8, no. 3, p. 392. DOI 10.3390/jcm8030392. MDPI AG (Clinical Study)
- Chondro-Gide® IFU 2019, Geistlich Pharma AG
- FICKERT, S. et al., 2017, Biologic Reconstruction of Full Sized Cartilage Defects of the Hip: A Guideline from the DGOU Group “Clinical Tissue Regeneration” and the Hip Committee of the AGA. Zeitschrift für Orthop.die und Unfallchirurgie. 2017. Vol. 155, no. 06, p. 670-682. DOI 10.1055/s-0043-116218. Georg Thieme Verlag KG (Guideline).
- FONTANA, A. and DE GIROLAMO, L., 2015, Sustained 5-year benefit of autologous matrix-induced chondrogenesis for femoral acetabular impingement-induced chondral lesions compared with microfracture treatment. The Bone & Joint Journal. 2015. Vol. 97-B, no. 5, p. 628-635. DOI 10.1302/0301-620x.97b5.35076. British Editorial Society of Bone & Joint Surgery (Clinical study).
- KAISER, N., et al. Clinical results 10 years after AMIC in the knee. Swiss Med Wkly, 2015, 145 (Suppl 210), 43S. (Clinical study).
- DE GIROLAMO, L., et al., 2018, Acetabular Chondral Lesions Associated With Femoroacetabular Impingement Treated by Autologous Matrix-Induced Chondrogenesis or Microfracture: A Comparative Study at 8-Year Follow-Up. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2018. Vol. 34, no. 11, p. 3012-3023. DOI 10.1016/j.arthro.2018.05.035. Elsevier BV (Clinical study).
- MANCINI, D., and FONTANA, A., 2014, Five-year results of arthroscopic techniques for the treatment of acetabular chondral lesions in femoroacetabular impingement. International Orthopaedics. 2014. Vol. 38, no. 10, p. 2057-2064. DOI 10.1007/s00264-014-2403-1. Springer Science and Business Media LLC (Clinical study).
Referenzen
- MARQUEZ-LARA, A. et al., 2016, Arthroscopic Management of Hip Chondral Defects: A Systematic Review of the Literature. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2016. Vol. 32, no. 7, p. 1435-1443. DOI 10.1016/j.arthro.2016.01.058. Elsevier BV (Review).
- SCHIAVONE PANNI, A., et al. Good clinical results with autologous matrix-induced chondrogenesis (AMIC) technique in large knee chondral defects. Knee Surg Sports Traumatol Arthrosc, 2018 Apr 26(4):1130-36 (Clinical study).
- WALTHER, M., et al. Scaffold based reconstruction of focal full thickness talar cartilage defects. Clinical Research on Foot & Ankle, 2013, 1-5. (Clinical study).
- Geistlich Pharma AG data on file (Pre-clinical Study)
- GILLE, J., et al. Cell-Laden and Cell-Free Matrix-Induced-Chondrogenesis versus Microfracture for the Treatment of Articular Cartilage Defects: A Histological and Biomechanical Study in Sheep. Cartilage OnlineFirst, January 7, 2010, doi:10.1177/1947603509358721 (Pre-clinical study)
- KRAMER, J., et al. In vivo matrix-guided human mesenchymal stem cells. Cell Mol Life Sci, Mar 2006, 3(5), 616-626. (Clinical study)
- FONTANA, A. and DE GIROLAMO, L., 2015, Sustained 5-year benefit of autologous matrix-induced chondrogenesis for femoral acetabular impingement-induced chondral lesions compared with microfracture treatment. The Bone & Joint Journal. 2015. Vol. 97-B, no. 5, p. 628-635. DOI 10.1302/0301-620x.97b5.35076. British Editorial Society of Bone & Joint Surgery (Clinical study).
- DE GIROLAMO, L., et al., Autologous Matrix-Induced Chondrogenesis (AMIC) and AMIC Enhanced by Autologous Concentrated Bone Marrow Aspirate (BMAC) Allow for Stable Clinical and Functional Improvements at up to 9 Years Follow-Up: Results from a Randomized Controlled Study. Journal of Clinical Medicine. 2019. Vol. 8, no. 3, p. 392. DOI 10.3390/jcm8030392. MDPI AG (Clinical Study)
- Chondro-Gide® IFU 2019, Geistlich Pharma AG
- FICKERT, S. et al., 2017, Biologic Reconstruction of Full Sized Cartilage Defects of the Hip: A Guideline from the DGOU Group “Clinical Tissue Regeneration” and the Hip Committee of the AGA. Zeitschrift für Orthop.die und Unfallchirurgie. 2017. Vol. 155, no. 06, p. 670-682. DOI 10.1055/s-0043-116218. Georg Thieme Verlag KG (Guideline).
- FONTANA, A. and DE GIROLAMO, L., 2015, Sustained 5-year benefit of autologous matrix-induced chondrogenesis for femoral acetabular impingement-induced chondral lesions compared with microfracture treatment. The Bone & Joint Journal. 2015. Vol. 97-B, no. 5, p. 628-635. DOI 10.1302/0301-620x.97b5.35076. British Editorial Society of Bone & Joint Surgery (Clinical study).
- KAISER, N., et al. Clinical results 10 years after AMIC in the knee. Swiss Med Wkly, 2015, 145 (Suppl 210), 43S. (Clinical study).
- DE GIROLAMO, L., et al., 2018, Acetabular Chondral Lesions Associated With Femoroacetabular Impingement Treated by Autologous Matrix-Induced Chondrogenesis or Microfracture: A Comparative Study at 8-Year Follow-Up. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2018. Vol. 34, no. 11, p. 3012-3023. DOI 10.1016/j.arthro.2018.05.035. Elsevier BV (Clinical study).
- MANCINI, D., and FONTANA, A., 2014, Five-year results of arthroscopic techniques for the treatment of acetabular chondral lesions in femoroacetabular impingement. International Orthopaedics. 2014. Vol. 38, no. 10, p. 2057-2064. DOI 10.1007/s00264-014-2403-1. Springer Science and Business Media LLC (Clinical study).
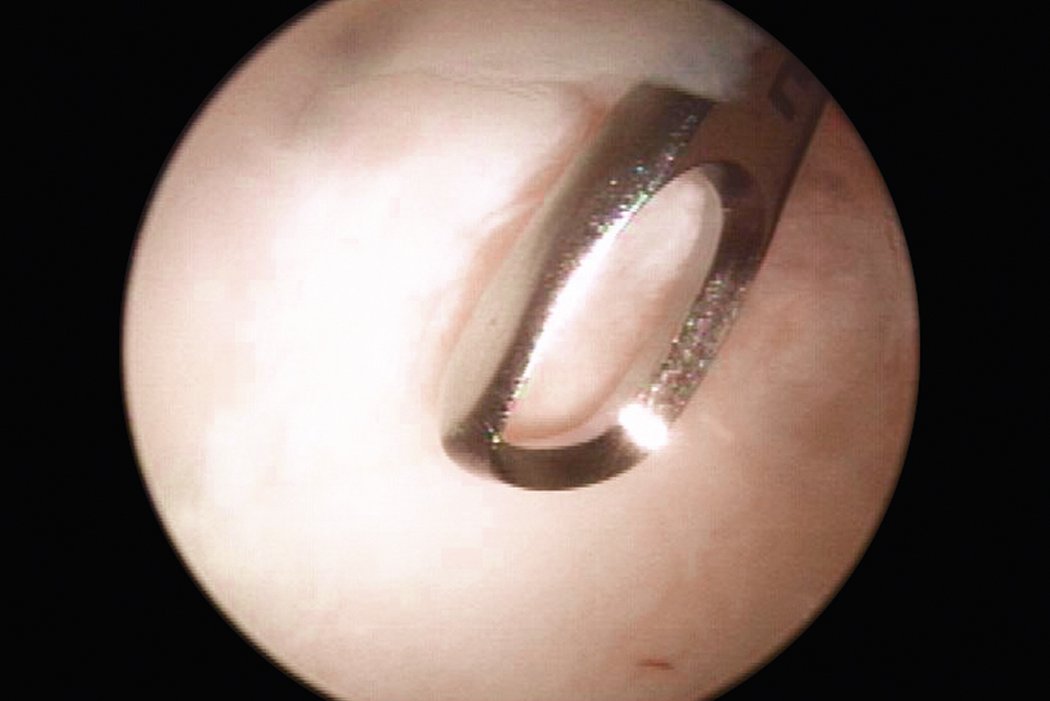
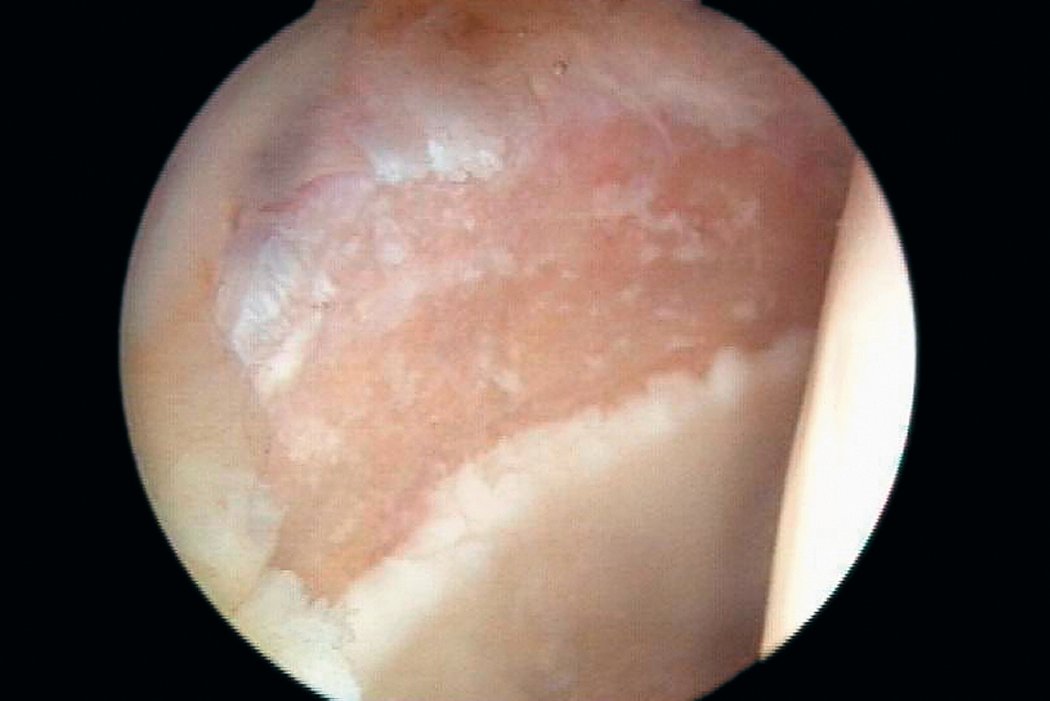
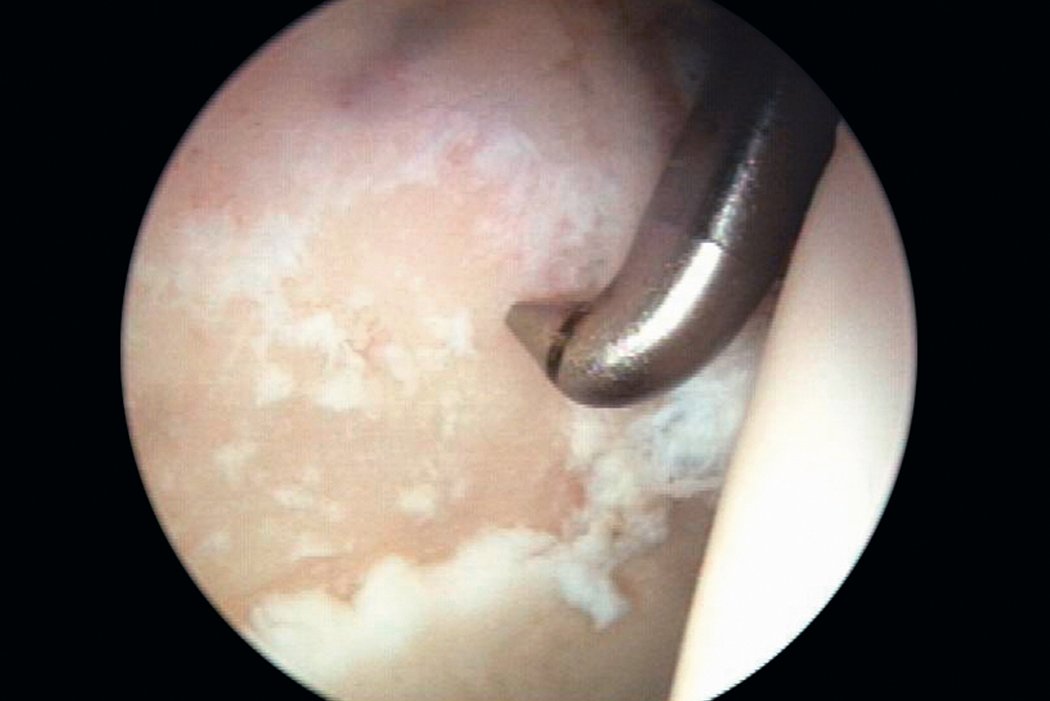
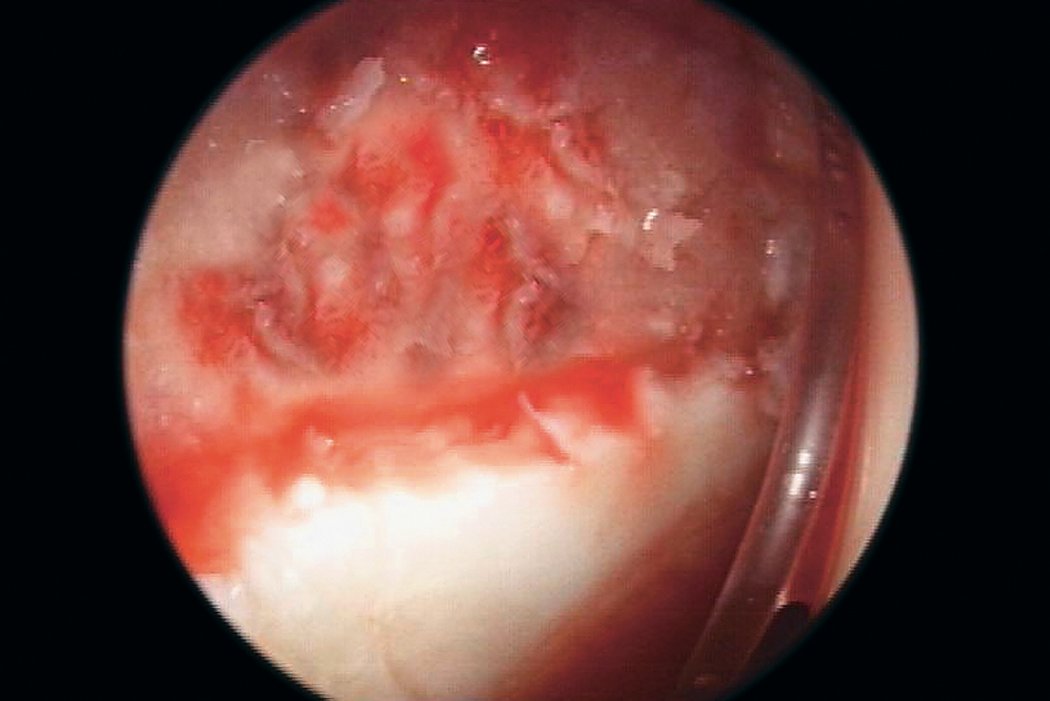
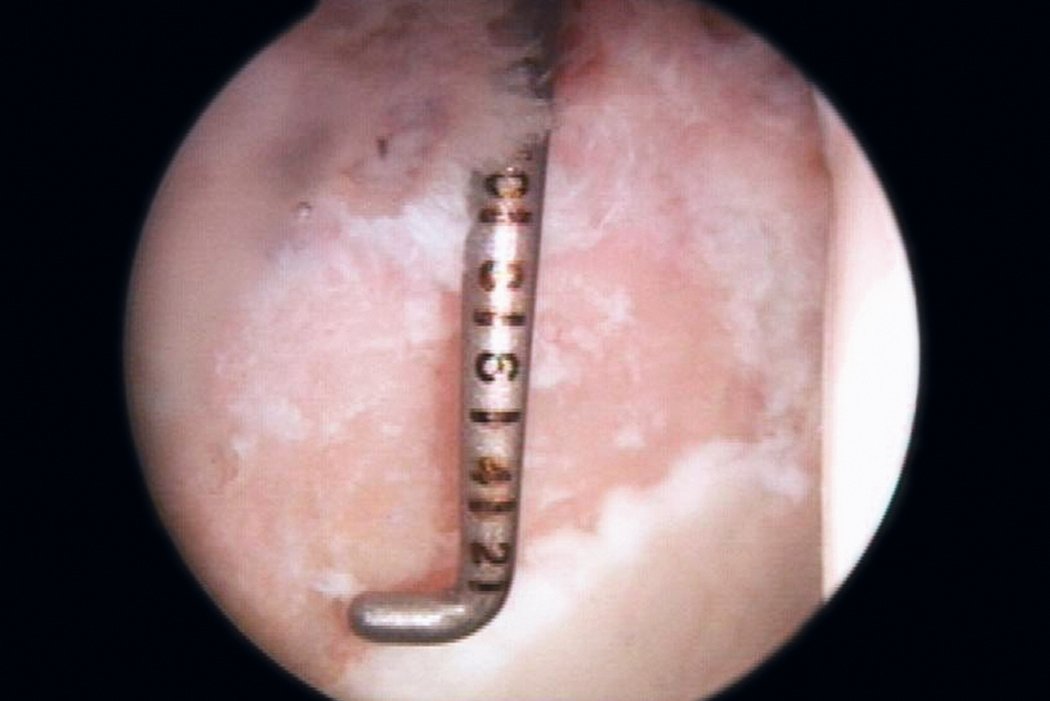
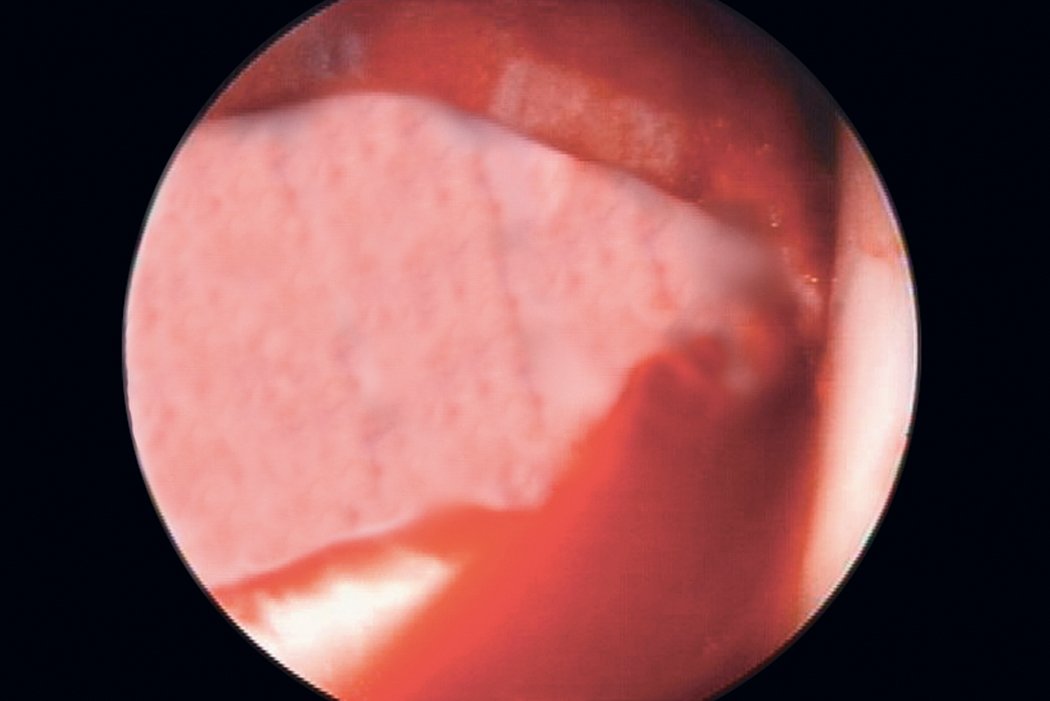
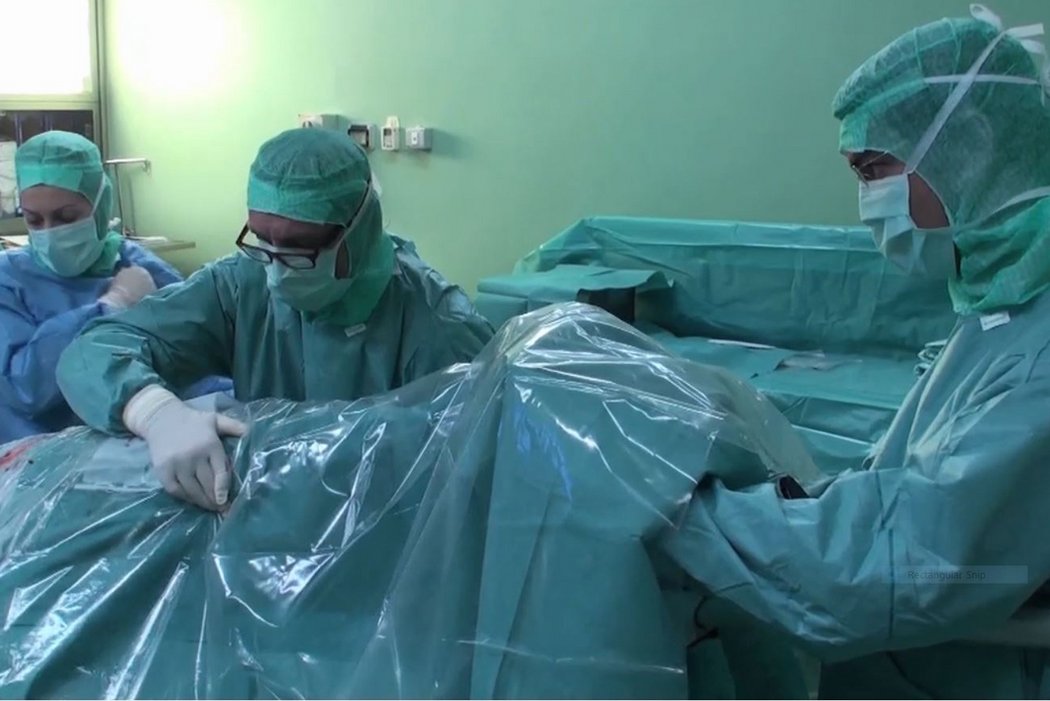
AMIC® in der Hüft-Operationstechnik nach Dr. Andrea Fontana

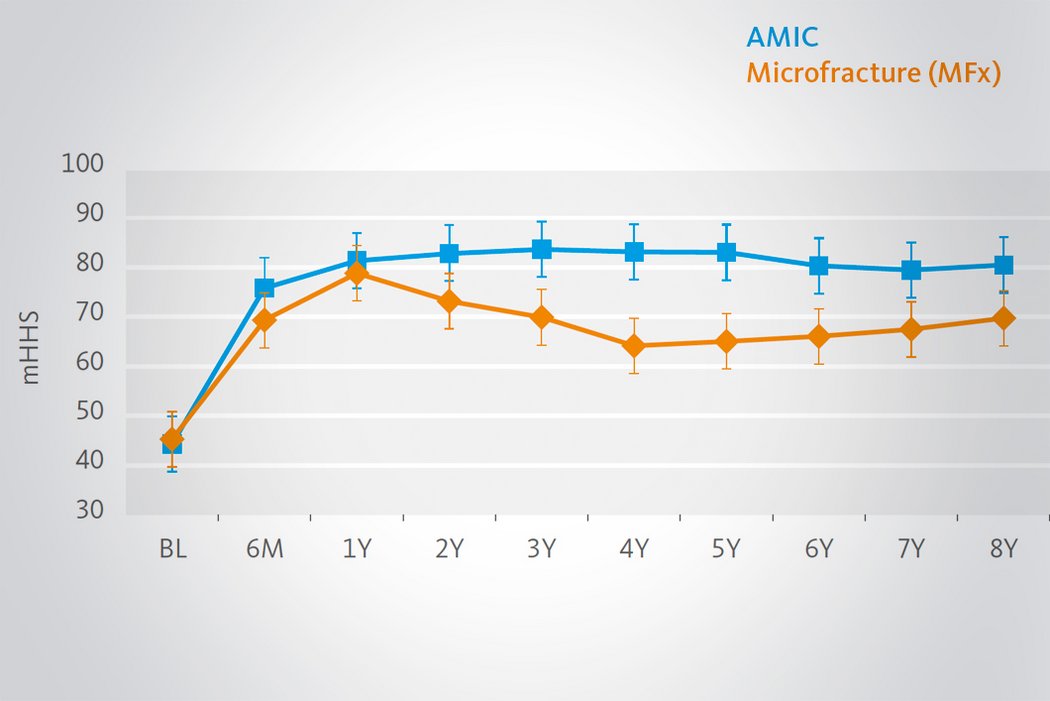
Klinische Ergebnisse - AMIC® in der Hüfte
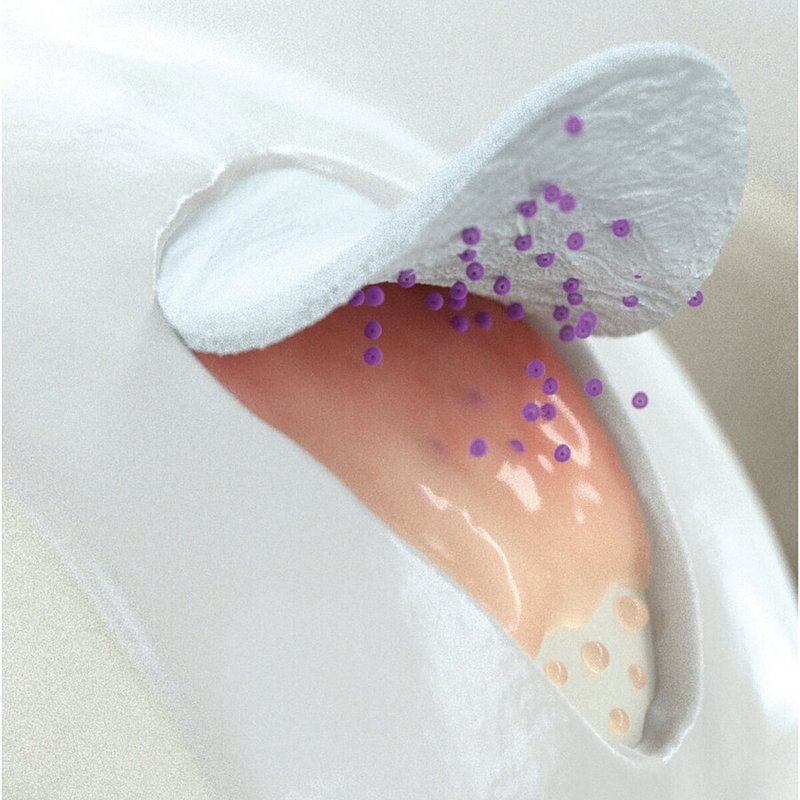
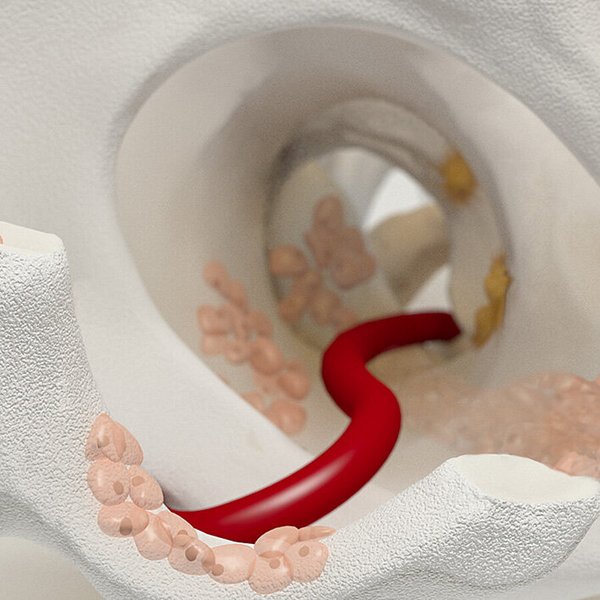
Wie Chondro-Gide® wirkt
Chondro-Gide® bildet eine Schutzschicht, die dafür sorgt, dass die aus dem Knochen freigesetzten oder in den Defekt eingeführten Zellen an Ort und Stelle bleiben. Die Membran unterstützt die regenerative Behandlung von chondralen und osteochondralen Läsionen. In der Anfangsphase des Heilungsprozesses werden der Defekt und die Zellen zunächst mit Chondro-Gide® abgedeckt. Nach etwa 4 Monaten9 wird die Matrix dann resorbiert und durch körpereigenes Gewebe ersetzt.
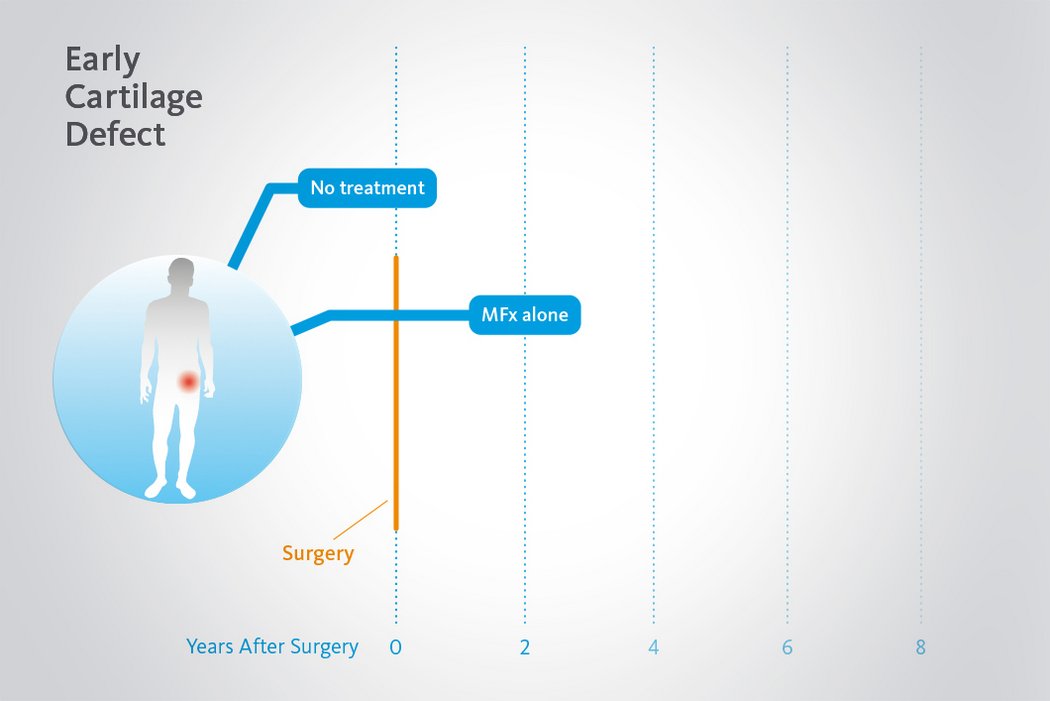
Langzeitvorteile von AMIC® Chondro-Gide® gegenüber MFx allein
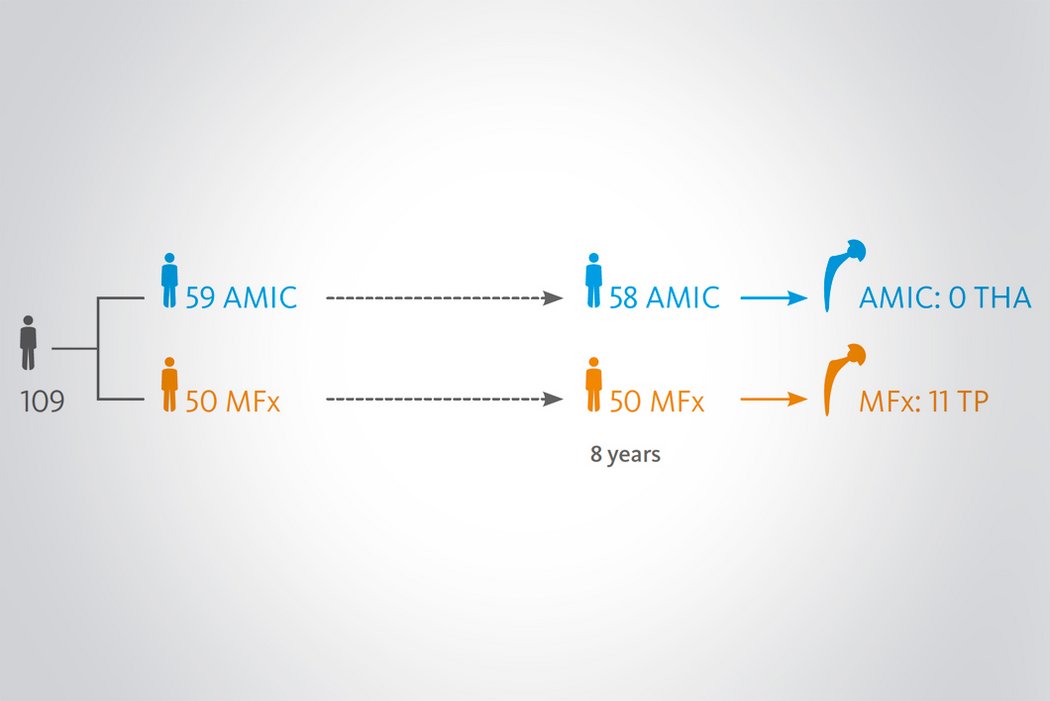
Der Einsatz von Chondro-Gide® in der Hüfte ist gut etabliert. Daten bis zu 8 Jahre nach der OP zeigen deutlich die Langzeitvorteile von AMIC® Chondro-Gide® im Vergleich zu MFx allein bei azetabulären Defekten10,11,12. In einer Studie, die arthroskopische MFx allein mit AMIC® Chondro-Gide® vergleicht, untersuchten Fontana et al. 109 Patienten. Patienten mit chondralen Defekten in der Hüfte, die mit FAIs assoziiert wurden, wurden mit AMIC® Chondro-Gide® oder MFx behandelt. Zwischen den zwei Patientengruppen gab es keine signifikanten Unterschiede betreffend Alter oder durchschnittliche Defektgrösse.
References
- MARQUEZ-LARA, A. et al., 2016, Arthroscopic Management of Hip Chondral Defects: A Systematic Review of the Literature. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2016. Vol. 32, no. 7, p. 1435-1443. DOI 10.1016/j.arthro.2016.01.058. Elsevier BV (Review).
- SCHIAVONE PANNI, A., et al. Good clinical results with autologous matrix-induced chondrogenesis (AMIC) technique in large knee chondral defects. Knee Surg Sports Traumatol Arthrosc, 2018 Apr 26(4):1130-36 (Clinical study).
- WALTHER, M., et al. Scaffold based reconstruction of focal full thickness talar cartilage defects. Clinical Research on Foot & Ankle, 2013, 1-5. (Clinical study).
- Geistlich Pharma AG data on file (Pre-clinical Study)
- GILLE, J., et al. Cell-Laden and Cell-Free Matrix-Induced-Chondrogenesis versus Microfracture for the Treatment of Articular Cartilage Defects: A Histological and Biomechanical Study in Sheep. Cartilage OnlineFirst, January 7, 2010, doi:10.1177/1947603509358721 (Pre-clinical study)
- KRAMER, J., et al. In vivo matrix-guided human mesenchymal stem cells. Cell Mol Life Sci, Mar 2006, 3(5), 616-626. (Clinical study)
- FONTANA, A. and DE GIROLAMO, L., 2015, Sustained 5-year benefit of autologous matrix-induced chondrogenesis for femoral acetabular impingement-induced chondral lesions compared with microfracture treatment. The Bone & Joint Journal. 2015. Vol. 97-B, no. 5, p. 628-635. DOI 10.1302/0301-620x.97b5.35076. British Editorial Society of Bone & Joint Surgery (Clinical study).
- DE GIROLAMO, L., et al., Autologous Matrix-Induced Chondrogenesis (AMIC) and AMIC Enhanced by Autologous Concentrated Bone Marrow Aspirate (BMAC) Allow for Stable Clinical and Functional Improvements at up to 9 Years Follow-Up: Results from a Randomized Controlled Study. Journal of Clinical Medicine. 2019. Vol. 8, no. 3, p. 392. DOI 10.3390/jcm8030392. MDPI AG (Clinical Study)
- Chondro-Gide® IFU 2019, Geistlich Pharma AG
- FICKERT, S. et al., 2017, Biologic Reconstruction of Full Sized Cartilage Defects of the Hip: A Guideline from the DGOU Group “Clinical Tissue Regeneration” and the Hip Committee of the AGA. Zeitschrift für Orthop.die und Unfallchirurgie. 2017. Vol. 155, no. 06, p. 670-682. DOI 10.1055/s-0043-116218. Georg Thieme Verlag KG (Guideline).
- FONTANA, A. and DE GIROLAMO, L., 2015, Sustained 5-year benefit of autologous matrix-induced chondrogenesis for femoral acetabular impingement-induced chondral lesions compared with microfracture treatment. The Bone & Joint Journal. 2015. Vol. 97-B, no. 5, p. 628-635. DOI 10.1302/0301-620x.97b5.35076. British Editorial Society of Bone & Joint Surgery (Clinical study).
- KAISER, N., et al. Clinical results 10 years after AMIC in the knee. Swiss Med Wkly, 2015, 145 (Suppl 210), 43S. (Clinical study).
- DE GIROLAMO, L., et al., 2018, Acetabular Chondral Lesions Associated With Femoroacetabular Impingement Treated by Autologous Matrix-Induced Chondrogenesis or Microfracture: A Comparative Study at 8-Year Follow-Up. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2018. Vol. 34, no. 11, p. 3012-3023. DOI 10.1016/j.arthro.2018.05.035. Elsevier BV (Clinical study).
- MANCINI, D., and FONTANA, A., 2014, Five-year results of arthroscopic techniques for the treatment of acetabular chondral lesions in femoroacetabular impingement. International Orthopaedics. 2014. Vol. 38, no. 10, p. 2057-2064. DOI 10.1007/s00264-014-2403-1. Springer Science and Business Media LLC (Clinical study).
AMIC® vs MACI
Eine 5-Jahres-Studie von Mancini und Fontana verglich das Ergebnis von AMIC® Chondro-Gide® und matrixinduzierter autologer Chondrozytenimplantation (MACI) bei der Behandlung von mittelgrossen azetabulären chondralen Defekten.13 AMIC® bietet weitere Vorteile als einzeitiges, minimal-invasives Verfahren, das die Gesamtbehandlungsdauer reduzieren und die Sterblichkeit minimieren kann.
Für weitere Details zu Chondro-Gide®, Operationstechniken und klinischen Erkenntnissen laden Sie die Broschüre herunter.
References
- MARQUEZ-LARA, A. et al., 2016, Arthroscopic Management of Hip Chondral Defects: A Systematic Review of the Literature. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2016. Vol. 32, no. 7, p. 1435-1443. DOI 10.1016/j.arthro.2016.01.058. Elsevier BV (Review).
- SCHIAVONE PANNI, A., et al. Good clinical results with autologous matrix-induced chondrogenesis (AMIC) technique in large knee chondral defects. Knee Surg Sports Traumatol Arthrosc, 2018 Apr 26(4):1130-36 (Clinical study).
- WALTHER, M., et al. Scaffold based reconstruction of focal full thickness talar cartilage defects. Clinical Research on Foot & Ankle, 2013, 1-5. (Clinical study).
- Geistlich Pharma AG data on file (Pre-clinical Study)
- GILLE, J., et al. Cell-Laden and Cell-Free Matrix-Induced-Chondrogenesis versus Microfracture for the Treatment of Articular Cartilage Defects: A Histological and Biomechanical Study in Sheep. Cartilage OnlineFirst, January 7, 2010, doi:10.1177/1947603509358721 (Pre-clinical study)
- KRAMER, J., et al. In vivo matrix-guided human mesenchymal stem cells. Cell Mol Life Sci, Mar 2006, 3(5), 616-626. (Clinical study)
- FONTANA, A. and DE GIROLAMO, L., 2015, Sustained 5-year benefit of autologous matrix-induced chondrogenesis for femoral acetabular impingement-induced chondral lesions compared with microfracture treatment. The Bone & Joint Journal. 2015. Vol. 97-B, no. 5, p. 628-635. DOI 10.1302/0301-620x.97b5.35076. British Editorial Society of Bone & Joint Surgery (Clinical study).
- DE GIROLAMO, L., et al., Autologous Matrix-Induced Chondrogenesis (AMIC) and AMIC Enhanced by Autologous Concentrated Bone Marrow Aspirate (BMAC) Allow for Stable Clinical and Functional Improvements at up to 9 Years Follow-Up: Results from a Randomized Controlled Study. Journal of Clinical Medicine. 2019. Vol. 8, no. 3, p. 392. DOI 10.3390/jcm8030392. MDPI AG (Clinical Study)
- Chondro-Gide® IFU 2019, Geistlich Pharma AG
- FICKERT, S. et al., 2017, Biologic Reconstruction of Full Sized Cartilage Defects of the Hip: A Guideline from the DGOU Group “Clinical Tissue Regeneration” and the Hip Committee of the AGA. Zeitschrift für Orthop.die und Unfallchirurgie. 2017. Vol. 155, no. 06, p. 670-682. DOI 10.1055/s-0043-116218. Georg Thieme Verlag KG (Guideline).
- FONTANA, A. and DE GIROLAMO, L., 2015, Sustained 5-year benefit of autologous matrix-induced chondrogenesis for femoral acetabular impingement-induced chondral lesions compared with microfracture treatment. The Bone & Joint Journal. 2015. Vol. 97-B, no. 5, p. 628-635. DOI 10.1302/0301-620x.97b5.35076. British Editorial Society of Bone & Joint Surgery (Clinical study).
- KAISER, N., et al. Clinical results 10 years after AMIC in the knee. Swiss Med Wkly, 2015, 145 (Suppl 210), 43S. (Clinical study).
- DE GIROLAMO, L., et al., 2018, Acetabular Chondral Lesions Associated With Femoroacetabular Impingement Treated by Autologous Matrix-Induced Chondrogenesis or Microfracture: A Comparative Study at 8-Year Follow-Up. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2018. Vol. 34, no. 11, p. 3012-3023. DOI 10.1016/j.arthro.2018.05.035. Elsevier BV (Clinical study).
- MANCINI, D., and FONTANA, A., 2014, Five-year results of arthroscopic techniques for the treatment of acetabular chondral lesions in femoroacetabular impingement. International Orthopaedics. 2014. Vol. 38, no. 10, p. 2057-2064. DOI 10.1007/s00264-014-2403-1. Springer Science and Business Media LLC (Clinical study).