Geistlich Mucograft®
User Benefits
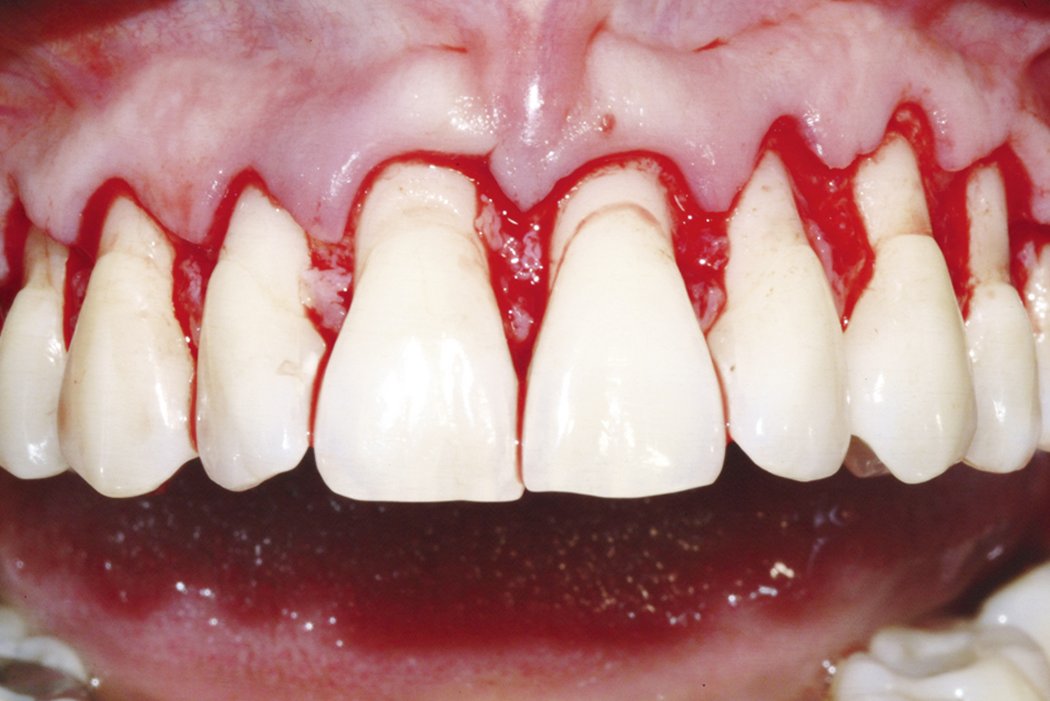
Geistlich Mucograft® is a collagen matrix designed specifically for soft-tissue regeneration in the oral cavity1. It is indicated for gaining keratinized tissue2,3,4 and for recession coverage5,6.
Geistlich Mucograft® provides an alternative to autologous soft-tissue grafts2,4,5,6. Painful harvesting of tissue is avoided, benefiting patients and clinicians alike.
Leading clinicians rely on Geistlich Mucograft®:
- No harvest-site morbidity2,3,5-7.
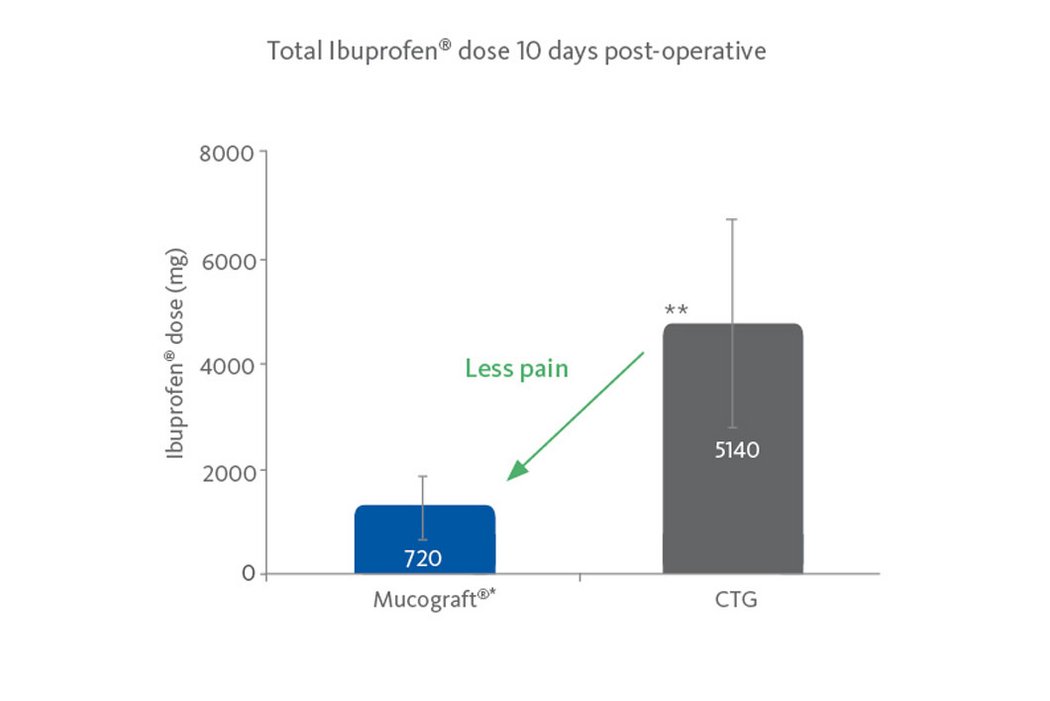
- Less pain compared with autologous grafts2,4.
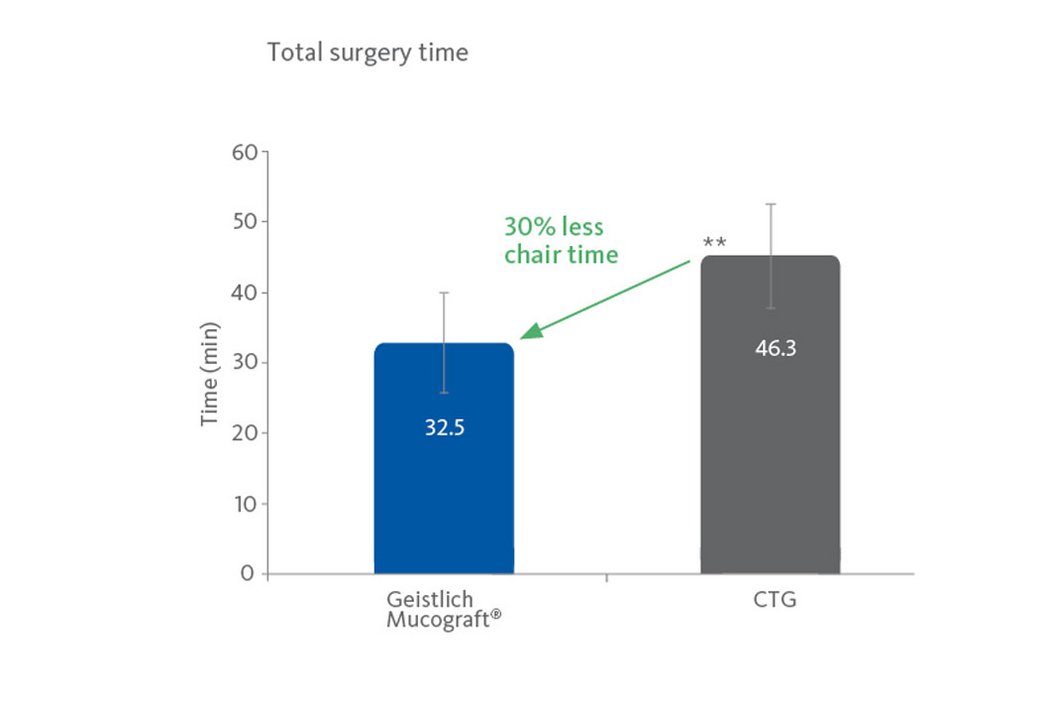
- Reduced surgical chair-time compared with autologous grafts2-6.
- Natural soft-tissue colour and texture match5,8,9.
- Early vascularization and good soft-tissue ingrowth10,11.
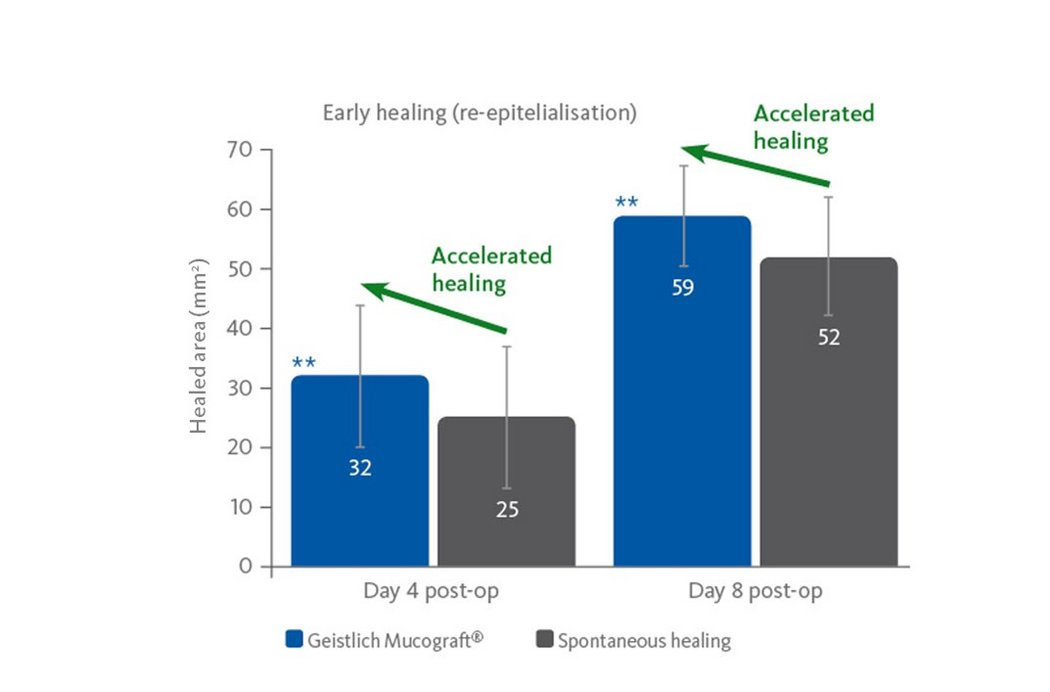
- Good wound healing, also in open situations2.
- Easy handling2 and application in a dry state.
Geistlich Mucograft® consists of two structures: the compact structure provides stability while allowing open healing; the spongy structure supports blood clot stabilization and ingrowth of soft-tissue cells.
References:
- Biocompatibility according to ISO 10993-1. Data on file, Geistlich Pharma AG, Wolhusen, Switzerland.
- Sanz M, et al.: J Clin Periodontol 2009; 36(10): 868-76. (clinical study)
- Konter U, et al.: Deutsche Zahnärztliche Zeitschrift 2010; 65: 723-30. (clinical study)
- Lorenzo R, et al.: Clin Oral Impl Res 2012; 23(3): 316-24. (clinical study)
- McGuire MK & Scheyer ET: J Periodontol 2010; 81(8): 1108-17. (clinical study)
- Cardaropoli D, et al.: J Periodontol 2012; 83(3): 321-28. (clinical study)
- Herford AS, et al.: J Oral Maxillofac Surg 2010; 68(7): 1463-70. (clinical study)
- Thoma, D, et al.: J Clin Periodontol 2012; 39(2): 157-65. (clinical study)
- Nevins M, et al.: Int J Periodontics Restorative Dent 2011; 31(4): 367-73. (clinical study)
- Ghanaati S, et al.: Biomed Mater. 2011 Feb; 6(1): 015010. (pre-clinical study)
- Rocchietta I, et al.: Int J Periodontics Restorative Dent. 2012; 32(1): e34-40. (pre-clinical study)
* Mucograft® (prototype) exhibited highly similar physical, mechanical and biological properties to the final product Geistlich Mucograft® differing only in the porcine collagen source used.
Application

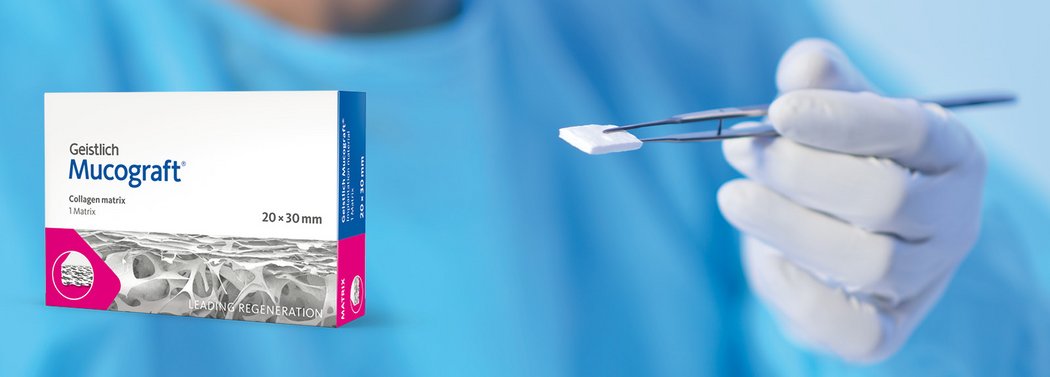
Geistlich Mucograft® is easy to handle compared with autologous soft-tissue grafts1:
- Unlimited availability and consistent quality.
- No need for pre-treatment or pre-hydration.
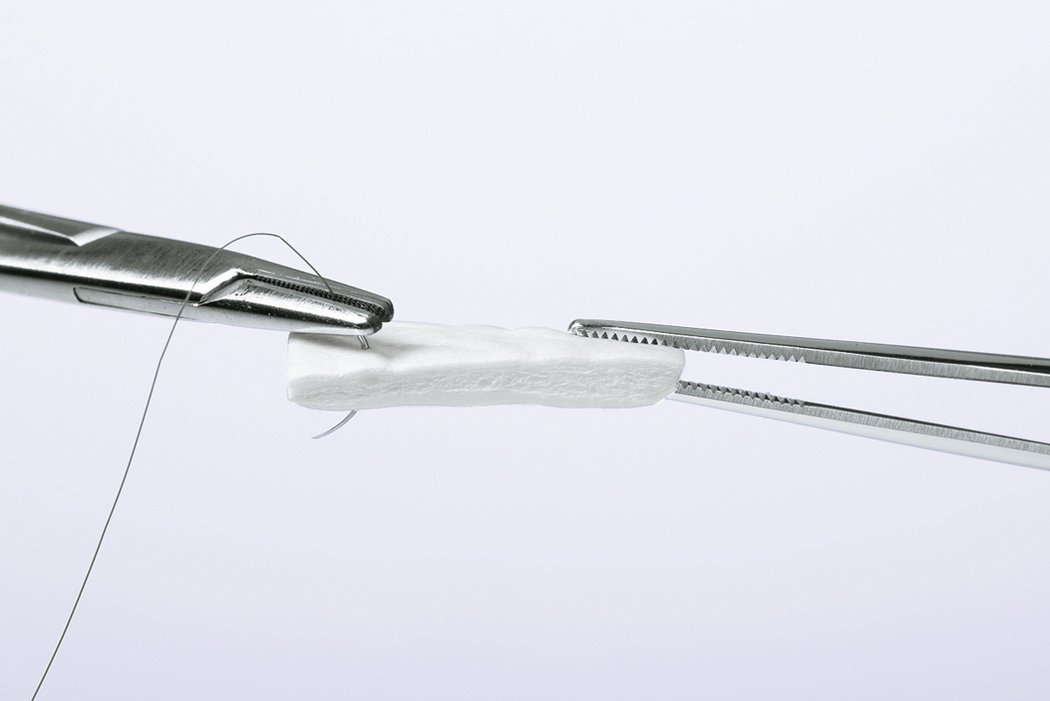
- Measure the defect and trim the matrix to the required shape.
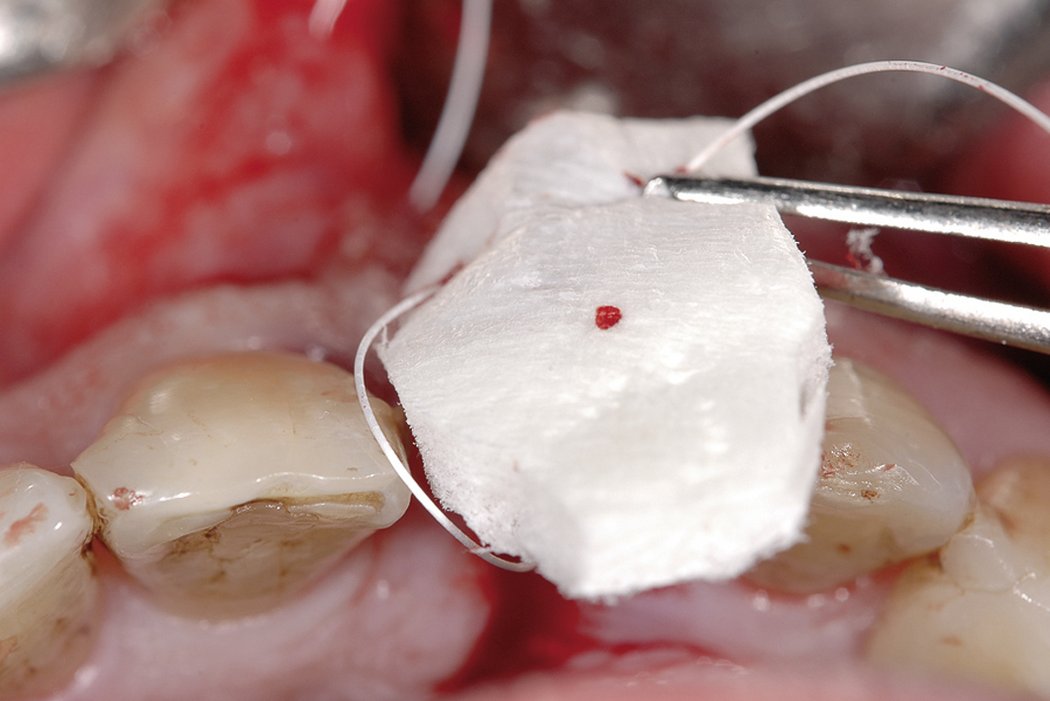
- Apply the dry Geistlich Mucograft® to the defect; it will moisten rapidly due to marked hydrophilicity.
- Position the compact structure facing outwards and the spongy structure towards the bone or periosteum.
- Soaked matrix adapts spontaneously to contours and adheres well to defect.
- The compact structure provides optimal suture pull-out strength.
References:
- Sanz M, et al.: J Clin Periodontol 2009; 36(10): 868-76. (clinical study)
- Abundo R & Corrente G: Chirurgia plastica parodontale – Trattamento estetico delle recessioni gengivali. ACME Edizioni, 2010. (book, clinical)
Product Range
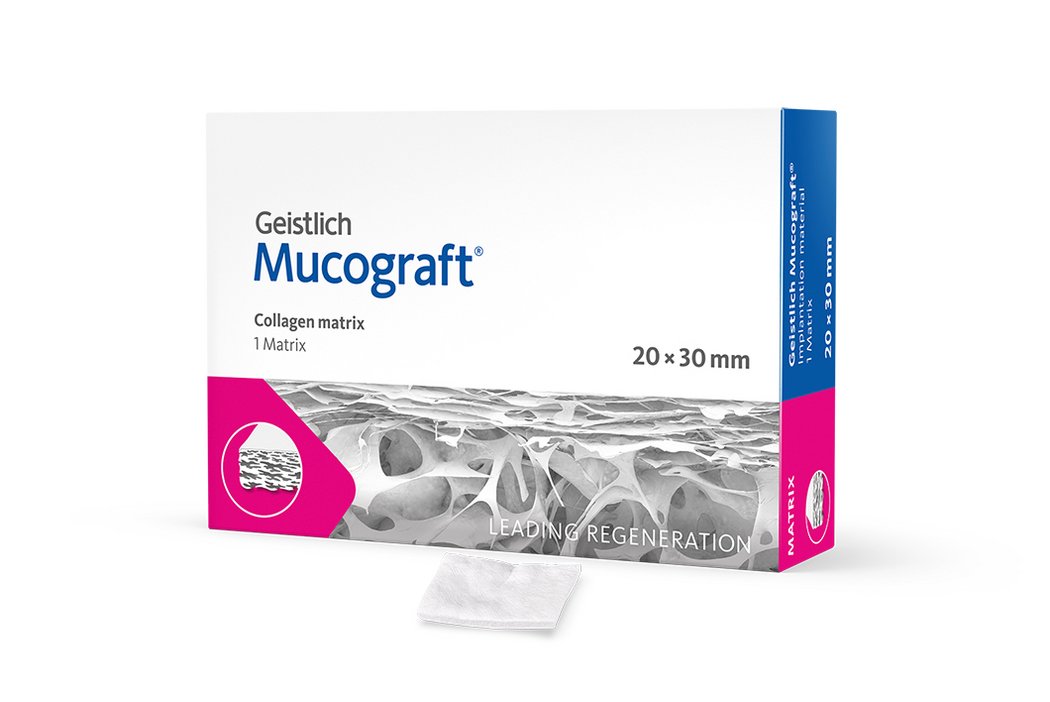
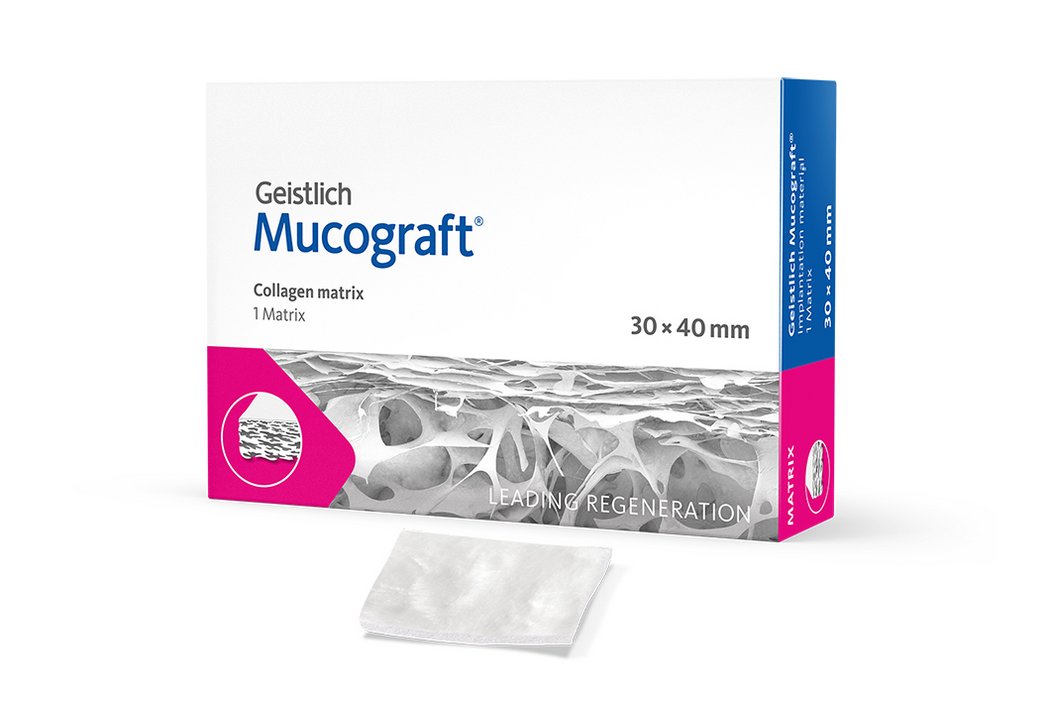
Geistlich Mucograft® consists of porcine collagen and is specifically designed for soft-tissue regeneration. The matrix is available in three sizes:
- 15 x 20 mm
- 20 x 30 mm
- 30 x 40 mm
Disclaimer: Mucograft is a registered trademark of Ed. Geistlich Söhne AG, Switzerland, in certain countries, including Australia, Brazil, China, France, Germany, Italy, Korea, United Kingdom, United States of America. This list is not exhaustive, and the trademark may not be registered in all countries for which Ed. Geistlich Söhne AG or its subsidiaries has localized websites. Use of the trademark on this country-specific website does not imply registration or endorsement in this particular country or region.