Geistlich TauroSept®
TauroSept CRBSI
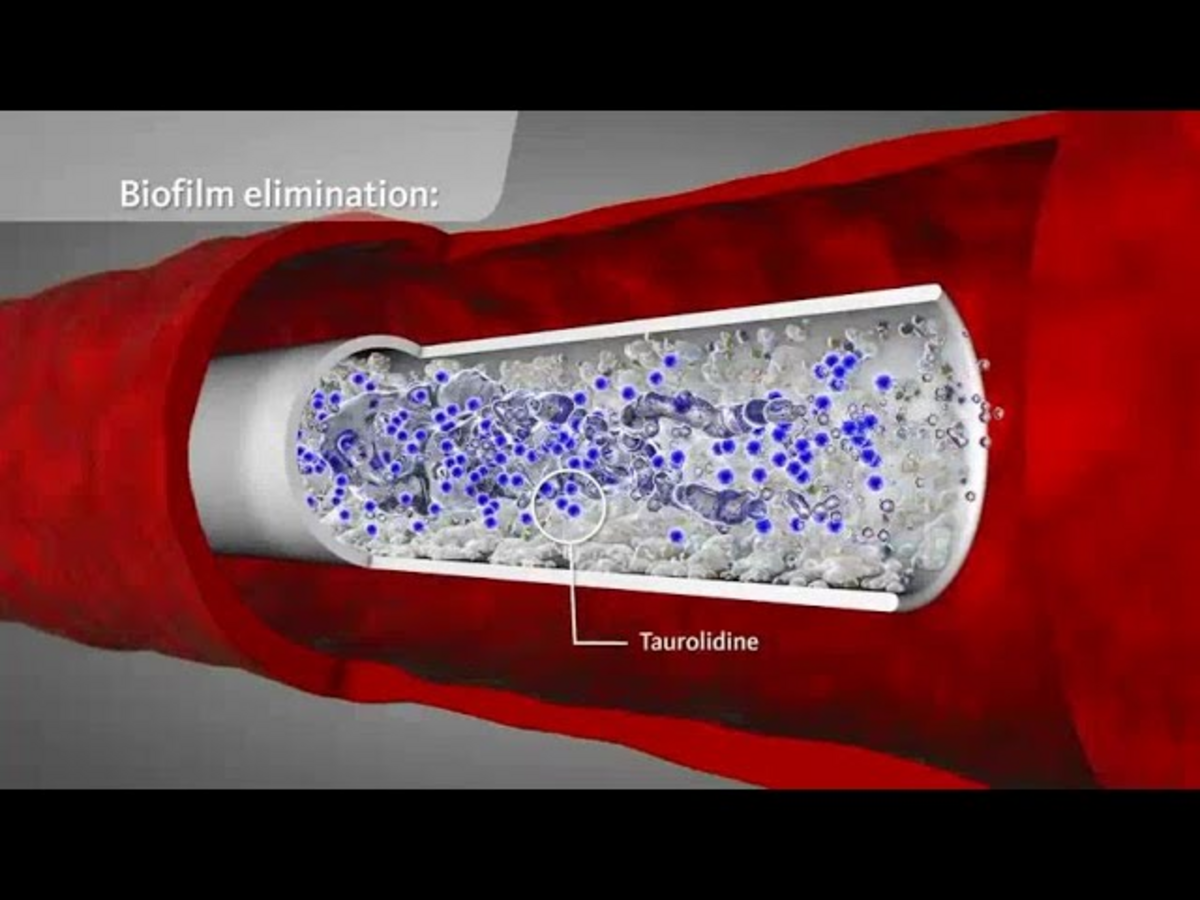
Optimal avoidance of infection and infection control are an absolute necessity when dealing with central vascular access devices (CVADs). Nevertheless, the incidence of CRBSI in Europe is 1.1 – 4.2 per 1000 catheter days despite the most varied preventive measures and the mortality of CRBSI is still between 5 and 25%.(1, 2, 3) The risk of CRBSI increases steadily depending on the duration of catheterisation. Already 24 hours after insertion of a CVAD, the vascular device can be coated with a biofilm. This typically consists of polysaccharides, fibrin, fibronectin or laminin and is formed by both microorganisms and endogenous substances.(4)
References:
- Mermel, L. A. (2001). “New technologies to prevent intravascular catheter-related bloodstream
- infections.” Emerg Infect Dis 7(2): 197–9.
- Munoz, P., E. Bouza, et al. (2004). “Clinical-epidemiological characteristics and outcome
- of patients with catheter-related bloodstream infections in Europe (ESGNI-006 Study).” Clin Microbiol Infect 10(9): 843-5.
- Tacconelli, E., G. Smith, et al. (2009). “Epidemiology, medical outcomes and costs of catheter-
- related bloodstream infections in intensive care units of four European countries: literature- and registry-based estimates.” J Hosp Infect 72(2): 97–103.
- Donlan, R. M., Costerton J. W. (2002). “Biofilms: Survival mechanisms of clinically relevant
- microorganisms.” CMR.15.2.167–193.
- Bouza, E., R. San Juan, et al. (2004). “A European perspective on intravascular catheter-related
- infections: report on the microbiology workload, aetiology and antimicrobial susceptibility (ESGNI-005 Study).” Clin Microbiol Infect 10(9): 838–42.
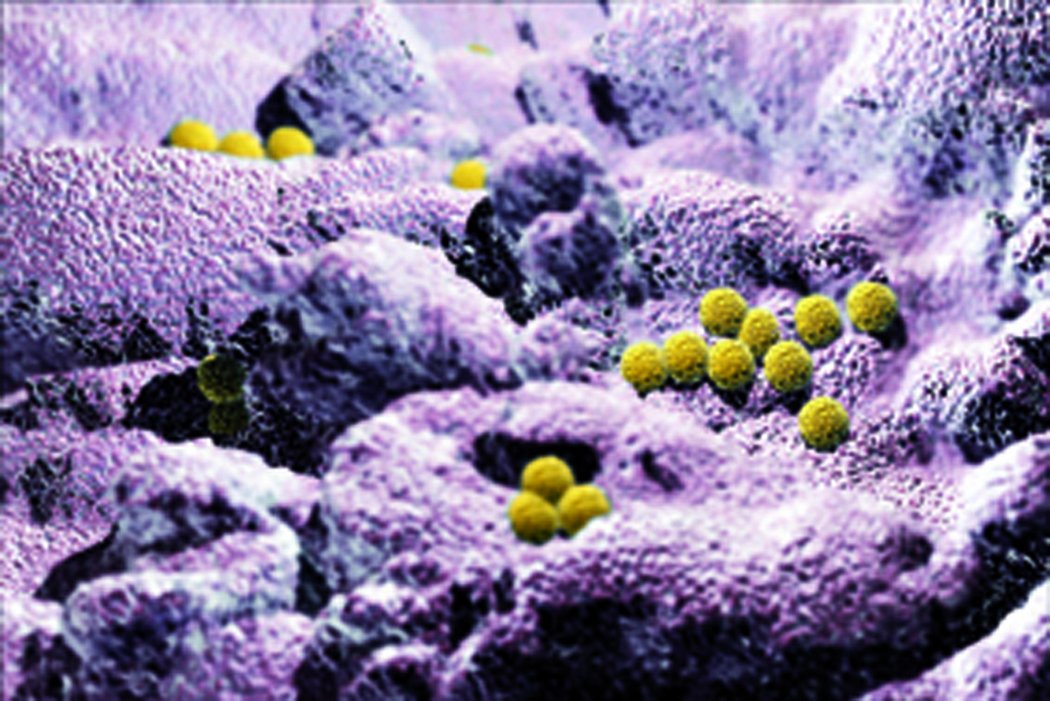
Microorganisms can reach the bloodstream via the outer surface of the CVAD or through the lumen; they lodge in the biofilm and are protected against immune mechanisms (phagocytosis, antibodies) and also partially against antibiotics. Gram-positive bacteria are responsible for about 50 – 70 % of CRBSI. In a European CRBSI prevalence study, gram-positive bacteria were detected in 71 %, gram-negative bacteria in 22 % and fungi in 7 %. The 5 most common microorganisms were coagulase-negative staphylococci, Staphylococcus aureus, Candida spp., Enterococcus spp. and Pseudomonas spp. (5)
References:
- Mermel, L. A. (2001). “New technologies to prevent intravascular catheter-related bloodstream
- infections.” Emerg Infect Dis 7(2): 197–9.
- Munoz, P., E. Bouza, et al. (2004). “Clinical-epidemiological characteristics and outcome
- of patients with catheter-related bloodstream infections in Europe (ESGNI-006 Study).” Clin Microbiol Infect 10(9): 843-5.
- Tacconelli, E., G. Smith, et al. (2009). “Epidemiology, medical outcomes and costs of catheter-
- related bloodstream infections in intensive care units of four European countries: literature- and registry-based estimates.” J Hosp Infect 72(2): 97–103.
- Donlan, R. M., Costerton J. W. (2002). “Biofilms: Survival mechanisms of clinically relevant
- microorganisms.” CMR.15.2.167–193.
- Bouza, E., R. San Juan, et al. (2004). “A European perspective on intravascular catheter-related
- infections: report on the microbiology workload, aetiology and antimicrobial susceptibility (ESGNI-005 Study).” Clin Microbiol Infect 10(9): 838–42.
User Benefits
The best prevention against the development of CRBSIs is observation of strict hygiene guidelines when inserting and manipulating a CVAD. In addition, the instillation of antimicrobial solutions like Geistlich TauroSept® into the device lumen (antimicrobial lock) has been shown to be clinically effective. (1, 2, 3, 4)
Geistlich TauroSept® is intended for instillation in CVADs between treatments in order to lock the catheter, to prevent bacterial and fungal growth leading to microbial infection in the CVAD lumen as well as to maintain device patency by avoiding clotting of blood.
Overview of Geistlich TauroSept®:
- Broad bactericidal and fungicidal spectrum
- Prevention of biofilm formation
- No development of bacterial resistance
- Favourable safety profile
References:
- Bisseling, T. M., M. C. Willems, et al. (2010). “Taurolidine lock is highly effective in preventing catheter-related bloodstream infections in patients on home parenteral nutrition: a heparin- controlled prospective trial.” Clin Nutr 29(4): 464–8.
- Jurewitsch, B. and K. N. Jeejeebhoy (2005). “Taurolidine lock: the key to prevention of recurrent catheter-related bloodstream infections.” Clin Nutr 24(3): 462–5.
- Olthof, E. D., M. W. Versleijen, et al. (2014). “Taurolidine Lock Is Superior to Heparin Lock in the Prevention of Catheter Related Bloodstream Infections and Occlusions.” PLoS One 9 (11): e111216.
- Sherertz, R. J., M. S. Boger, et al. (2006). “Comparative in vitro efficacies of various catheter lock solutions.” Antimicrob Agents Chemother 50(5): 1865–8.
Application
Instruction for use
Geistlich TauroSept® can be used with any vascular access device. Follow the manufacturer's instructions for the particular catheter utilized. Specific catheter volumes are associated with each device and must be strictly followed. Use of the device by patients requires proper training by authorized health care personnel. If legally required, the training must be documented.
- Before each use, Geistlich TauroSept® should be inspected for the presence of visible particles. Do not use Geistlich TauroSept® if particles are visible.
- Flush the catheter with 10 ml sterile physiological saline solution before instillation of TauroSept®.
- Disinfect the surface of the septum with a non-iodine based disinfectant immediately before using Geistlich TauroSept®. Transfer the required volume of Geistlich TauroSept® from the vial with a sterile syringe and fill the lumen of the catheter with Geistlich TauroSept®. Remove the needle from the vial cap.
- Allow Geistlich TauroSept® to remain inside the catheter for at least 30 minutes but no longer than 4 weeks. Geistlich TauroSept® can be used as adjuvant treatment in infected catheters. If treating an infected catheter allow Geistlich TauroSept® to remain inside the catheter for 12 hours and replace the product every 12 hours until the desired effect is achieved.
- Geistlich TauroSept® is not to be used for systemic injection. The solution should be withdrawn from the catheter before next use. If withdrawal of Geistlich TauroSept® is not possible for technical reasons, e.g. with totally implantable venous access systems (portacaths), or clinically not wanted, e.g. in parenteral nutrition, the flushing of Geistlich TauroSept® can be done without systemic effect.
Precautions
Patients
- Geistlich TauroSept® should be handled with caution in patients with known allergic predisposition and when patients are concomitantly treated with products which are known to interact with taurolidine.
Interactions
- Geistlich TauroSept® must not be mixed with oxidizing agents such as Dakin's solution (sodium hypochlorite), povidone iodine, or hydrogen peroxide because of oxidation to formic acid. The risk can be considered to be very low as these agents are not used in the catheter but only on the surrounding skin.
Device handling
- Geistlich TauroSept® must be instilled into the access device as described in the product's user manual. Failure to comply with these instructions may result in inadvertent systemic injection of the taurolidine solution.
- Geistlich TauroSept® solution must only be instilled once and the withdrawn residue must be disposed of. Reuse may result in decreased effectiveness of the device.
- Do not use Geistlich TauroSept® if the vial is damaged.
- Geistlich TauroSept® must be stored in a horizontal position at a controlled temperature of 15 to 25 °C. Do not refrigerate.
- Do not use if the package is damaged. If the package is opened and no date and time indicating first opening are legible on the vial, the product must not be used.
Presentation and package sizes
Each original pack contains 5 glass vials containing 6 ml or 10 ml of Geistlich TauroSept®. The product is sterile. Opened Geistlich TauroSept® vials can be stored for 48 hours and multiple withdrawal is possible. The content of a vial is for a single patient only and must be used within 48 hours after the first puncture. Write time and date when the vial was opened on the vial label. Re-sterilisation is not possible! Do not use if the packaging is damaged. If the packaging is opened and no date and time indicating first opening are legible on the vial, the product must not be used.
Scientific Evidence
Prevention and treatment of CVAD infections through broad anti-microbial activity
Geistlich TauroSept® contains the antimicrobial chemotherapeutic agent taurolidine 2 %. Unlike antibiotics, taurolidine acts via a chemical reaction with the microbial cell wall. Microbes are killed and resulting toxins are inactivated; the destruction time in vitro is 15–30 minutes. Taurolidine has an extremely broad antimicrobial and antimycotic spectrum, which also includes methicillin- and vancomycin-resistant bacteria (MRSA, VISA and VRE). (1, 2, 3, 4) Preventive instillation of Geistlich TauroSept® helps to avoid impending CVAD colonisation. (5, 6, 7) When CVAD colonisation already exists the microbial contamination can be eliminated by the therapeutic use of Geistlich TauroSept® in combination with systemic antibiotics. In many cases the removal of the CVAD can be avoided or delayed for a long time. (8, 9)
Reliability in long-term use due to the absence of development of microbial resistance
In many years of clinical use development of microbial resistance due to taurolidine has never been observed. Because of the special mechanism of action of taurolidine, in which a reaction with the microbial cell wall occurs directly, development of resistance is unlikely and not to be expected in contrast to antibiotics. (10, 11)
Interference with biofilm development through inhibition of microbial colonisation
Even at low concentrations, taurolidine causes a loss of microbial fimbriae and flagellae. Due to the change in the microbial surfaces, the capacity to form colonies is lost and the adhesion of microbes to the surfaces of epithelia and biomaterials is prevented. These antiadhesive properties of taurolidine counteract the biofilm formation. (12, 13)
Promotion of intraluminal haemodynamics by reducing local pathological coagulation phenomena
Depending on duration of exposure and concentration, taurolidine causes inhibition of staphylocoagulase-mediated coagulation, which cannot be influenced by heparin. The risk of pathological staphyloco-agulase-induced coagulation occurring especially at the CVAD tip is therefore reduced. (14)
Safety in use due to outstanding systemic tolerability
Taurolidine is also licensed as an active pharmaceutical ingredient for the local treatment of infections such as peritonitis; up to 200 ml of taurolidine 2 % are instilled daily into the abdominal cavity and absorbed fully through the peritoneum. So far, no systemic side effects have been identified. The safety of taurolidine has also been confirmed in clinical studies with long-term intravenous administration of high doses (up to 20 g daily).15 In the body, taurolidine is metabolised rapidly via the metabolites taurultam and methylol taurinamide, which also have an anti-microbial action, to taurine, an endogenous aminosulphonic acid, CO2 and H2O. Therefore, no toxic effects are known or expected in the event of accidental injection.16
References:
- Olthof, E. D., R. J. Rentenaar, et al. (2013). “Absence of microbial adaptation to taurolidine in patients on home parenteral nutrition who develop catheter related bloodstream infections and use taurolidine locks.” Clin Nutr 32 (4): 538–42.
- Olthof, E. D., R. Nijland, et al. (2015). “Microbiocidal effects of various taurolidine containing catheter lock solutions.” Clin Nutr 34 (2): 309–14.
- Torres-Viera, C., C. Thauvin-Eliopoulos, et al. (2000). “Activities of taurolidine in vitro and in experimental enterococcal endocarditis.” Antimicrob Agents Chemother 44(6): 1720-4.
- Traub, W. H., B. Leonhard, et al. (1993). “Taurolidine: in vitro activity against multiple-antibiotic-resistant, nosocomially significant clinical isolates of Staphylococcus aureus, Enterococcus faecium, and diverse Enterobacteriaceae.” Chemotherapy 39(5): 322–30.
- Bisseling, T. M., M. C. Willems, et al. (2010). “Taurolidine lock is highly effective in preventing catheter-related bloodstream infections in patients on home parenteral nutrition: a heparin- controlled prospective trial.” Clin Nutr 29(4): 464–8.
- Jurewitsch, B., T. Lee, et al. (1998). “Taurolidine 2 % as an antimicrobial lock solution prevention of recurrent catheter-related bloodstream infections.” JPEN J Parenter Enteral Nutr 22(4): 242–4.
- Olthof, E. D., M. W. Versleijen, et al. (2014). “Taurolidine Lock Is Superior to Heparin Lock in the Prevention of Catheter Related Bloodstream Infections and Occlusions.” PLoS One 9 (11): e111216.
- Koldehoff, M. and J. L. Zakrzewski (2004). “Taurolidine is effective in the treatment of central venous catheter-related bloodstream infections in cancer patients.” Int J Antimicrob Agents 24(5): 491–5.
- Weber, M., F. Meyer, et al. (2009). “[Spectrum of indications and perioperative management in i. v. port-a-cath explantation-alternative administration of taurolin in case of i. v. port-a-cath infection].” Zentralbl Chir 134(4): 350–6.
- Gorman, S. P., D. F. McCafferty, et al. (1987). “Reduced adherence of micro-organisms to human mucosal epithelial cells following treatment with Taurolin, a novel antimicrobial agent.” J Appl Bacteriol 62(4): 315–20.
- Blenkharn, J. I. (1987). “The antibacterial and anti-endotoxin activity of taurolidine in combination with antibiotics.” Surg Res Comm 2: 149–155.
- Blenkharn, J. I. (1988). “Sustained anti-adherence activity of taurolidine (Taurolin) and noxythiolin (Noxyflex S) solutions.” J Pharm Pharmacol 40(7): 509–11.
- Gorman, S. P., D. F. McCafferty, et al. (1987). “Reduced adherence of micro-organisms to human mucosal epithelial cells following treatment with Taurolin, a novel antimicrobial agent.” J Appl Bacteriol 62(4): 315–20.
- Reinmuller, J. (1999). “[The influence of taurolidine on physiological and pathological blood coagulation and implications for its use].” Zentralbl Chir 124 Suppl 4: 13–8.
- Gong, L., H. E. Greenberg, et al. (2007). “The pharmacokinetics of taurolidine metabolites in healthy volunteers.” J Clin Pharmacol 47(6): 697–703.
- Knight, B. I., G. G. Skellern, et al. (1981). “The characterisation and quantitation by high- performance liquid chromatography of the metabolites of taurolin.” Br J Clin Pharmacol 12 (3): 439–40.
Quality & Safety
Safety in use due to outstanding systemic tolerability
Taurolidine is also licensed as an active pharmaceutical ingredient for the local treatment of infections such as peritonitis; up to 200 ml of taurolidine 2 % are instilled daily into the abdominal cavity and absorbed fully through the peritoneum. So far, no systemic side effects have been identified. The safety of taurolidine has also been confirmed in clinical studies with long-term intravenous administration of high doses (up to 20 g daily). (1) In the body, taurolidine is metabolised rapidly via the metabolites taurultam and methylol taurinamide, which also have an anti-microbial action, to taurine, an endogenous aminosulphonic acid, CO₂ and H₂O. (2) Therefore, no toxic effects are known or expected in the event of accidental injection.
References:
- Gong, L., H. E. Greenberg, et al. (2007). “The pharmacokinetics of taurolidine metabolites in healthy volunteers.” J Clin Pharmacol 47(6): 697–703.
- Knight, B. I., G. G. Skellern, et al. (1981). “The characterisation and quantitation by high- performance liquid chromatography of the metabolites of taurolin.” Br J Clin Pharmacol 12 (3): 439–40.
Studies
Here you will find the links to the relevant studies for the use of Geistlich TauroSept®. These links lead to external websites. Please note that some studies can only be downloaded in connection with a registration on the respective website.
- Activities of Taurolidine In Vitro and in Experimental Enterococcal Endocarditis
- Taurolidine: In vitro Activity against Mulitple-Antibiotic-Resistance
- Absence of microbial adaptation to taurolidine in patients on home parenteral nutrition who develop catheter related bloodstream infections and use taurolidine locks
- Taurolidine is effective in the treatment of central venous catheter-related bloodstream infections in cancer patients
- Indikationsspektrum und perioperatives Management bei i.v.-Portsystemexplantation - Alternative Taurolingabe bei i. v.-Portsysteminfektion
- The effect of taurolidine on experimental thrombus formation
- Hemmung der Staphylokokken-Koagulase durch Taurolin
- The Pharmacokinetics of Taurolidine Metabolites in Healthy Volunteers
- Taurolidine Lock is iuperior to Heparin Lock in the Prevention of Catheter Related Bloodstream Infections and Occlusions
- Incidence of central venous catheter related bloodstream infections in andults and children on home parenteral nutrition: Heparin versus Taurolidine catheter lock
- Taurolidine lock is highly effective in preventing catheter-related bloodstream infections in patients on home parenteral nutrition
- Taurolidine lock: The key to prevention of recurrent catheter-related bloodstream infections
- Randomised clinical trial: 2% taurolidine versus 0.9% saline locking in patients on home parenteral nutrition Clinical and economic impact of the taurolidine lock on home parenteral nutrition
- ESPEN guidelines on chronic intestinal failure in adults
- KRINKO Prävention von Infektionen, die von Gefäßkathetern ausgehen